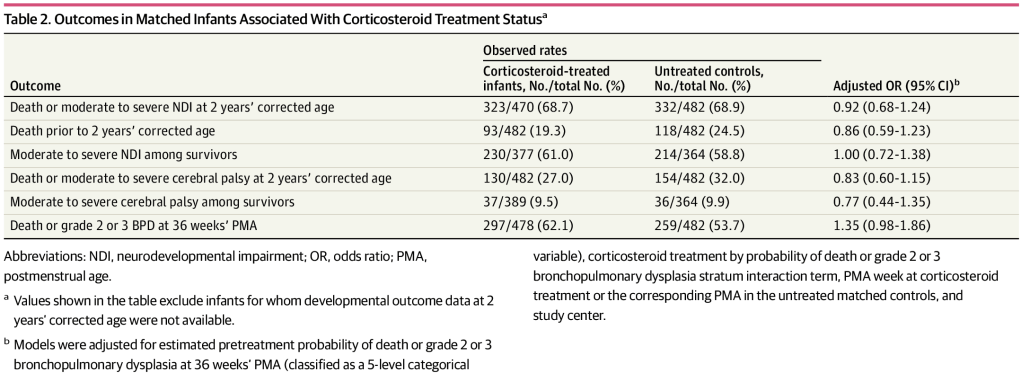
As this series of posts have all had something to do with prevention of Necrotising Enterocolitis, I thought I’d write about some recent articles which refer to one of the risk factors for NEC, the use of acid suppression medications.
I’ve written previously about the risks of proton pump inhibitors (ppi) in young infants. They are a group of medications (along with Histamine receptor blockers) that I almost never prescribe. The commonest reason given for prescribing them seems to be gastro-oesphageal reflux, but of course they have no effect on reflux, just on the pH of the refluxed liquid, which is not necessarily a beneficial effect. If a baby had acidic reflux oesophagitis then maybe blocking acid production would be beneficial, but most reflux in newborn infants is non-acid, either neutral or alkaline. In fact, at least one animal study shows that ppis cause relaxation of the lower oesophageal sphincter, which might increase the risk of reflux!
A number of recent epidemiologic publications point out the complications associated with altering the gastro-intestinal physiology of a large number of babies. And the numbers are indeed large, prevalence of use in the NICU is not clear, but in France and in Scandinavia a substantial, and increasing, proportion of all young infants are exposed to ppi medications, somewhere between 2 and 5% of all infants!
We also should remember that we are really poor at diagnosing reflux. The only reliable way to know if a baby has an abnormal frequency of reflux is by impedance oesophageal recordings (multiple intraluminal impedance, mii), and a new publication has, yet again, shown that there is no correlation between a baby being irritable, or having arching episodes, and the presence of reflux events. (Njeh M, et al. The Irritable Infant in the Neonatal Intensive Care Unit: Risk Factors and Biomarkers of Gastroesophageal Reflux Disease. J Pediatr. 2023:113760). In this retrospective study they report over 500 babies who had pH-mii studies, and over 40,000 GER events with nearly 40,000 arching or irritability events. Their conclusion is quite clear ;
Acid GER disease is unlikely the primary cause of arching/irritability and empiric treatment should not be used when arching/irritability is present. Prematurity and neurological impairment may be more likely the cause of the arching/irritability. Arching/irritability may not be a concern in orally feeding infants.
So what are the complications of ppi use? The most immediately important in the NICU population is an increased risk of sepsis. This is true in older infants in the community (Lassalle M, et al. Proton Pump Inhibitor Use and Risk of Serious Infections in Young Children. JAMA Pediatr. 2023), in a nation-wide cohort study from France. It is even more striking among children in the PICU (Goyer I, et al. Proton Pump Inhibitor Use and Associated Infectious Complications in the PICU: Propensity Score Matching Analysis. Pediatr Crit Care Med. 2022;23(12):e590-e4), in this propensity score matched analysis, among children in the PICU, in Caen in Normandy,, who mostly received the ppi for stress ulcer prophylaxis, the risk of nosocomial infections was NINE times higher if they had received a ppi.
The other complication which has been confirmed in a recent study is an increase in the risk of fractures, this study is a propensity matched national epidemiologic study from the USA (Achler T, et al. Association of early-life exposure to acid-suppressive therapy and fractures during childhood: a retrospective cohort study. Arch Dis Child. 2023). This is probably due to effects of acid suppression on calcium absorption, which have been shown to increase osteoporosis risk in the adult.
Other studies from the last few years show an association of ppi use with the development of asthma, and with subsequent development of Coeliac disease, and the development of a variety of allergic diseases.
To return to the title of the post, does acid suppression increase the risk of NEC? PPI medications cause major changes to the intestinal microbiome (Levy EI, et al. The effects of proton pump inhibitors on the microbiome in young children. Acta Paediatr. 2020), and have been associated with LOS and NEC (See the following studies Manzoni P, et al. Exposure to Gastric Acid Inhibitors Increases the Risk of Infection, Patil UP, et al. Efficacy of and potential morbidities associated with the use of antacid medications, and this review article (Tan J, et al. A Review of Histamine-2 Receptor Antagonist and Proton Pump Inhibitor Therapy for Gastroesophageal Reflux Disease in Neonates and Infants. Paediatr Drugs. 2023;25(5):557-76). As there are almost no RCTs of these agents in the preterm infant, there is no reliable evidence of the size of the risk, but as that review article points out there is no evidence of any benefit either. The epidemiologic studies suggest that there is no benefit, including among babies with a clinical diagnosis of reflux.
One situation in which a ppi is frequently prescribed is after Gastro-oesophageal fistula repair. Oesphageal dysmotility is universal after such surgery, and there is a risk of developing anastomotic strictures. A ppi or another antacid medication are often prescribed after surgery, just in case the infant has acid reflux, and in case the reflux might increase the risk of stricture. A recent publication from my centre (Righini Grunder F, et al. Should Proton Pump Inhibitors be Systematically Prescribed in Patients With Esophageal Atresia After Surgical Repair? J Pediatr Gastroenterol Nutr. 2019;69(1):45-51) shows that routine prescription of a ppi does not prevent formation of strictures, and that many babies were probably receiving them for other symptoms, such as those due to tracheomalacia, for which they are probably ineffective.
A final concern, ppi use induces hyperplasia of the parietal, acid-producing cells, by a secondary increase in gastrin production. Which means that there is often HYPERacidity when then are eventually stopped. Hence the title of the article from which this image is taken “Evidence That Proton-Pump Inhibitor Therapy Induces the Symptoms it Is Used to Treat“. They note in that article that Histamine-2 receptor blockade does the same thing.

My take home message? Don’t.
Don’t prescribe acid suppression therapy for suspected reflux.
Don’t prescribe acid suppression therapy for proven reflux, unless you can show acid oesophagitis
Don’t prescribe acid suppression therapy for prophylaxis against upper GI bleeding.
Don’t prescribe acid suppression therapy for preterm infants at any risk of NEC.