Two important new studies of the use of very early routine surfactant, compared to later selective surfactant if necessary. The first I will discuss is the one that didn’t seem to improve any important clinical outcomes (Murphy MC, et al. Prophylactic Oropharyngeal Surfactant for Preterm Newborns at Birth: A Randomized Clinical Trial. JAMA Pediatr. 2023 the POPART trial). 252 infants of less than 29 weeks (mean GA 26 weeks) were randomized prior to birth in 9 university hospitals in 6 European countries, co-ordinated by Colm O’Donnell in Dublin. The intervention was the instillation of 120 mg of Poractant into the pharynx for infants <26 weeks, and 240 mg for those 26-28, this was done without any suctioning, and prior to any positive pressure, ideally before clamping of the cord at 30 to 60 seconds after delivery. Only a small number of babies were protocol violations, being intubated outside of protocol defined indications. After the oro-pharyngeal surfactant, babies followed standard stabilisation, including intubation if required, and any baby thought to need surfactant was treated with the usual surfactant dose thereafter (either by intubation or LISA).
The primary outcome was intubation for respiratory failure in the 1st 120 hours of life, with fairly objective criteria, even though the intervention was, unsurprisingly, unmasked. As you can see here, there isn’t a hint of a difference between groups.
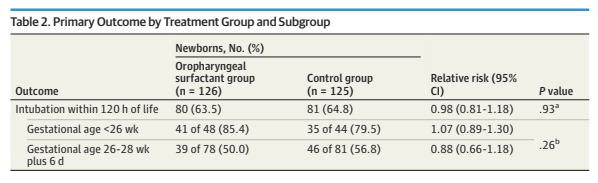
The only difference in secondary outcomes was an increase in pneumothoraces in the surfactant group, 17% vs 6%, not likely to be a random difference. Clinical BPD (70 vs 69%) and physiologic BPD were also just about identical, there was a minor difference in NEC, favouring the control group, and in home oxygen, favouring the surfactant group. Mortality was identical also, 18% in each group.
The rationale for the trial was based on previous pre-clinical data in rabbits showing that the administration method does lead to pulmonary surfactant deposition, and an old RCT in 328 babies of 25 to 29 weeks GA, with a dry powder surfactant which is no longer available, called ALEC (Artifical Lung Expanding Compound, which was developed by Colin Morley) in the Ten centre trial of artificial surfactant in very premature babies. (Ten Centre Study Group. Br Med J (Clin Res Ed). 1987;294(6578):991-6). In that study the prophylactic administration was performed in the delivery room, in a similar way to the POPART trial. In the Ten Centre trial, mortality was lower with surfactant. ALEC was a mixture of two phospholipids, DPPC and PG, and was eventually taken off the market as it was, overall, somewhat less effective than liquid surfactants containing protein.
Why ALEC would work, and lead to lower mortality, but poractant would not, and lead to increased pneumothorax, is not clear to me. Clearly, in the last 35 years many things have changed in neonatology, (I have witnessed all of them!) the Ten Centre group studied 328 babies of 25 to 29 weeks gestation, of whom 19% of the ALEC group and 30% of the controls died, with an overall mean GA of just under 28 weeks. Unfortunately there are some problems with the study design of that trial, it started as a much smaller pilot trial published in the widely circulated journal (!) known as “Colloids and Surfaces”, the results of which were published after about 35 babies of 25 to 29 weeks were reported, and there were 5 deaths in the control group, and 0 in the ALEC group. The later publication in the BMJ appears to have included and re-reported the outcomes of those pilot trial babies, as well as a much larger group added on after the initial benefit was shown. Routine early CPAP was not typically used in the Ten Centre study, perhaps that is why early prophylactic ALEC surfactant was effective, in comparison to standard care, which did not include routine early CPAP.
The mortality is overall about 50% higher in the old study, despite a substantially lower GA in the new trial, demonstrating some of the amazing improvements in survival over this time period. Overall respiratory and ICU management is so much better, that there are no apparent benefits from this intra-pharyngeal prophylactic approach. The controls in the older study did not receive routine CPAP, but in both control groups in the 2 new studies, controls routinely were supported with CPAP.
One thing which is not mentioned in the POPART manuscript, or in the protocol, is the use of caffeine, which although frequently given early, is not often given in very early life.
That is one of the 2 major differences between PROPART and CaLI, the routine administration of intravenous caffeine in the 1st 2 hours of life, the other difference being direct intra-tracheal surfactant administration by the LISA procedure, after the caffeine.
In the CaLI trial, which is unfortunately not open access (Katheria A, et al. Caffeine and Less Invasive Surfactant Administration for Respiratory Distress Syndrome of the Newborn. NEJM Evidence. 2023;2(12)), it was funded by Chiesi, I would have thought they could pay for open access as well! 180 babies between 24 and <29 weeks were randomized. The protocol was as briefly outlined above, Caffeine and CPAP versus Caffeine, LISA, and CPAP. To be enrolled, babies had to be breathing and stable at 5 to 60 minutes of age, if enrolled, babies were then weighed and had IV access inserted. LISA followed at least 5 minutes after the 20 mg/kg load of caffeine citrate. In the CPAP group, early postnatal caffeine was also given, which was intended to be before 2 hours of age in both groups.
Randomization was performed at an average of 7 minutes of age, the caffeine was given at a median of about 50 to 60 minutes, and the LISA performed in the LISA group at a median of 1.5 hours, IQR 0.9 to 2. The primary outcome of the trial was the diagnosis of respiratory failure in the first 72 hours of life, the criteria for which were an FiO2 of >40%, respiratory acidosis with a PCO2 >65 on 2 gases, or lots of apneas.
The primary outcome was dramatically reduced by Caffeine plus LISA, compared to Caffeine alone. 23% vs 53%, the benefit was very similar in the 2 GA strata, 44% vs 80% in the 24 to 26 wk group and 14% vs 51% in the 27 to 29 weeks group.
I am actually a bit confused about what exactly was the primary outcome. In the text of the document it is written, “The primary outcome of the trial was the frequency of neonates requiring endotracheal intubation or meeting respiratory failure criteria between the two groups (caffeine and LISA vs. caffeine and CPAP) within the first 72 HoL” but then the tables just mention intubation, and babies in the caffeine plus CPAP group could have had LISA without necessarily being intubated. In the table showing the primary outcome results,
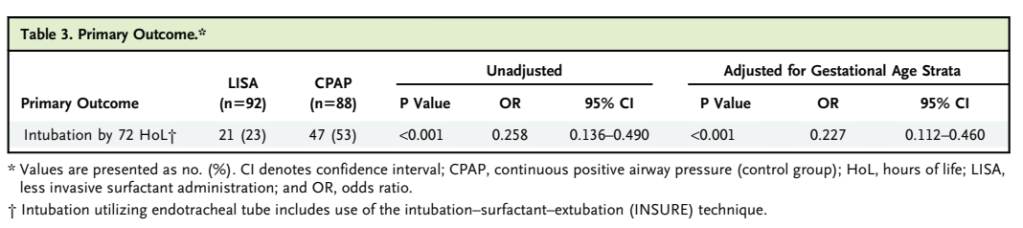
the implication is that all the babies counted were actually intubated, even though LISA was permitted in the caffeine alone group. LISA is not much used in the USA currently, so perhaps all the “intubation or respiratory failure” babies were actually intubated. There were only 3 deaths, all in the CPAP without LISA group.
Among other outcomes, there was no sign of adverse effects, and the proportion of babies in oxygen at 36 weeks fell from 35 to 21% in the LISA group. I previously discussed the presentation of these results at the PAS earlier this year, and how Anup Katheria, the first author and PI, put the results together with the OPTIMIST trial. There aren’t yet any data on more clinically important long-term respiratory outcomes with the CaLI approach, but follow up is planned, and, if such outcomes are improved, we will have to figure out the best way to implement the approach. I’d love to figure out how to reduce the discomfort/pain of laryngoscopy, without any respiratory depression, and be more comfortable at performing LISA in the 50% of babies who would never have needed intubation.
CORRECTION: post changed 15 December 2023: The post initially read that the protocol violations in POPART were of babies “being intubated before the POPART intervention”. Colm O’Donnell has informed me that I misinterpreted the violations, the protocol violations were all babies who received the intervention, but were intubated outside of protocol indications, such as because they were “very small” or “needing a lot of oxygen”. As Colm also points out, if there was insufficient surfactant reaching the lungs to improve lung function, then how could the increase in pneumothoraces be blamed on the intervention? (I paraphrase), he is right of course, it doesn’t make much sense, so I guess the difference in pneumothorax rates may just be an accidental occurrence. As for caffeine timing, it isn’t known exactly when the babies in POPART received their caffeine, but they probably mostly received it quite early….









I’d like to ask Prof Barrington for his opinion on both the CALi trial and the Optimist trial. In basic sense both these trials are examining the effect of early surfactant vs delayed surfactant. I thought we already knew that early surfactant is beneficial compared to delayed surfactant. If these trials set out to examine if LISA as a technique is superior or not then the trial design should be surfactant via LISA vs surfactant via ETT! I’d be very grateful for Prof’s opinion on this issue.
Thank you