This new publication is not a complete systematic review, but is a review of the history, design and outcomes of the oxygen saturation targeting trials, and of the early stopping of 2 trials. Stenson BJ. Oxygen Saturation Targets for Extremely Preterm Infants after the NeOProM Trials. Neonatology. 2016;109(4):352-8. It includes a meta-analysis of the primary outcomes of the trials, including mortality, disability, and certain of the components of those outcomes. The only difference between the groups was in mortality, which led to a significant difference in the combined primary outcome, of death or disability at 18 to 24 months. In case you can’t get access to the full text, here are 2 small parts of the publication, the mortality data, showing a 16% increase in mortality with lower saturations, and no heterogeneity between trials:
Ben Stenson discusses the impacts of the changes in oximeter calibration algorithm, and shows the before and after mortality data for those studies where the algorithm was changed part way through :
Perhaps because the separation between the saturation ranges was a little greater after the change, that is where the mortality difference is seen, in all the 3 trials where it was changed. There is also an increase in NEC (relative risk of 1.25), which is at least one of the causes of death that seems to have increased with the lower saturations.
One might ask why the SUPPORT trial, with only the old algorithm, showed a difference in mortality. I think it is possible that that was because of the antenatal consent and enrollment at birth, it may be that aiming for lower saturations is even more hazardous if you start the lower saturations immediately during the transition period.
Nevertheless the first graph shows that all of the data together, including both algorithms and all the trials, there is an increase in mortality.
The final conclusion is:
In trials conducted in a developed world setting with continuous SpO2 monitoring and protocols for screening for and treating ROP, targeting SpO2 below 90% in extremely preterm infants increased mortality and did not reduce the risk of blindness or other disabilities and cannot be recommended.


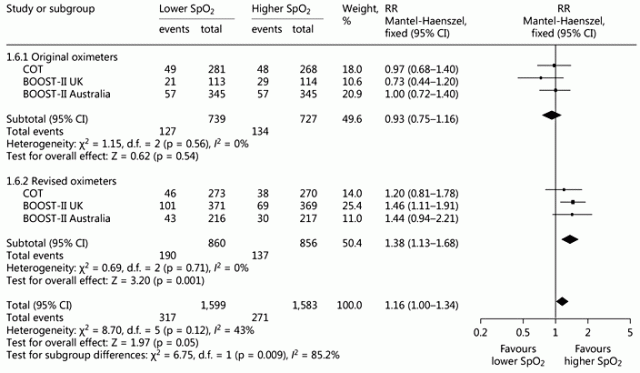








How about we use the ‘original oximeters’ and aim for lower sats target – then we would have no difference in mortality but less ROP.
Mark raises an important point. Does the ‘negative’ result for mortality in the 1466 infants managed with original oximeters mean (i) there was no clinically important difference or (ii) a clinically important difference may exist, but was not detected? The wide confidence interval (0.75 – 1.16) around the risk ratio for mortality in lower versus higher target infants supports the second explanation. As it’s consistent with either a 25% reduction or a 16% increase in mortality, it excludes neither substantial benefit nor substantial harm. [1,2]
The most reliable way to reduce the uncertainty around estimates of effects on mortality is through continuing international collaboration – as achieved by the pioneering NeOProM collaborators [3] – and even larger sample sizes. [2]
Incidentally, Professor Jonathan Morris, Melinda Cruz, I and others will be launching the ALPHA Collaboration (Advancing Large Perinatal trials for Health outcomes Assessment) for perinatal mega-trials at the upcoming Sydney International Update on Advances in Perinatal Care, to be held between 4-6 August. Colleagues can visit the website to view the program and register at
siu.ctc.usyd.edu.au
William Tarnow-Mordi
References:
1. Tarnow-Mordi WO, Healy MJ. Distinguishing between “no evidence of effect” and “evidence of no effect” in randomised controlled trials and other comparisons. Arch Dis Child 1999;80:210-1.
2. Tarnow-Mordi W, Cruz M, Morris J. Design and conduct of a large obstetric or neonatal randomized controlled trial. Semin Fetal Neonatal Med 2015;20:389-402.
3. Askie LM, Brocklehurst P, Darlow BA, Finer N, Schmidt B, Tarnow-Mordi W. NeOProM: Neonatal Oxygenation Prospective Meta-analysis Collaboration study protocol. BMC Pediatr 2011;11:6.
Although lower saturation targets were associated will less ROP treatment, the treatment is usually successful and there was no difference in blindness. The numbers were nearly identical. There were actually marginally more blind infants with lower targets. This was the case with the original oximeters. In a developed world setting with high quality screening and treatmenrt for ROP it is not worth risking a single death from hypoxia to avoid ROP.
I think Mark’s (cleverly-made) point is about the risk of spuriously “significant” findings in post hoc subgroup analyses.