The word “probiotic” is defined rather vaguely as a micro-organism which has beneficial health impacts. I think it is obvious that there is a huge difference between fungi that are found in the intestinal microbiome of adults, and the lactic acid bacteria which are major components in the young infant.
Even that term “lactic acid bacteria” includes organisms which are dramatically different. Lactobacilli, of the phylum Firmicutes (also called Bacillota), are gram positive rods which are facultative anaerobes, and have limited synthetic capacity, fermenting hexoses to produce lactic acid. Bifidobacteria are Bifid gram positive rods, hence the name, they are often portrayed as tiny little ‘Y’s, and are from a different phylum, the Actinobacteria (or Actinomycetota). They are obligate anaerobes, and have varying abilities to metabolise Hexoses, but remarkable abilities to metabolise oligosaccharides (Human Milk Oligosaccharides, HMOs) that are present in large quantities in breastmilk, but which humans lack the ability to digest.
The only reason these HMOs are present in breastmilk is to feed the Bifidobacteria, which, when they are established and reproduce, come to dominate the intestinal microbiome of the breastfed baby. In particular, a subspecies of B Longum, known as Bifidobacterium Longum ssp Infantis, is a micro-organism that seems to have co-evolved with humans, and is able to digest just about the entire range of HMOs, of which there may be over 200. (Underwood MA, et al. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res. 2015;77(1-2):229-35). HMO composition of human milk is variable, but B Infantis has 24 glycoside hydrolase genes and, alone among GI commensals, possesses sialidases and fucosidases allowing it to digest all types of HMOs.
I don’t for a minute think that breastmilk composition and B infantis evolved in this symbiotic manner in order to prevent NEC! But the GI tract of the full term newborn, who had a possibility of survival, is a haven for nasty pathogens, that can thrive if they have access to food, and which sometimes need access to iron. Hence the presence of Lactoferrin in substantial quantities in breastmilk, which binds iron to keep it out of the clutches of certain Gram negatives, and allows very high bioavailability of breastmilk iron, shuttling it into enterocytes, via specific human lactoferrin receptors, that strip off the iron and resecrete the lactoferrin. Hence the presence of those HMOs, which feed bifidobacteria but for which many pathogens, such as E Coli, Clostridia, Enterobacter and Staphylococci, completely lack the enzymes required to feed on them.
During the evolution of humanity it looks like the constant pressure to avoid GI and systemic infections, in order to survive to be able to pass on our genes, led to this symbiotic relationship between breastmilk and B Infantis. It led to the evolution of breastmilk that is packed with molecules that can only be utilised by Bifidobacteria, and specifically with a high degree of activity by B Infantis. B Infantis can inhibit the growth of other organisms, as well as starving them by eating up all the HMOs, and reduces inflammation by damping down the activity of the TLR4. TLR4 has an affinity for G negative LPS endotoxin, and seems (probably, I guess, by accident) to be overexpressed in the very immature bowel (Meng D, et al. Toll-like receptor-4 in human and mouse colonic epithelium is developmentally regulated: a possible role in necrotizing enterocolitis. Pediatr Res. 2015;77(3):416-24).
B Infantis also seems to decrease gut permeability and translocation of pathogens, at least in part by stabilising tight junction proteins. (Bergmann KR, et al. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol. 2013;182(5):1595-606.)
When there are a lot of B Infantis about, their metabolic activity leads to production of acids, lactate and acetate, and other short chain fatty acids. Which leads to a low stool pH. A fascinating study published 5 years ago (Henrick BM, et al. Elevated Fecal pH Indicates a Profound Change in the Breastfed Infant Gut Microbiome Due to Reduction of Bifidobacterium over the Past Century. mSphere. 2018;3(2):10.1128/msphere.00041-18) traced the changes in stool pH over the last century, as recorded in various publications, and showed that stool pH in breast fed babies used to be as low as 5, and has increased to as high as 6.5. There is a clear correlation between this increase and lower colonization by Bifidobacteria.
The intestinal protection afforded by this normal microbiome is the reason behind the use of probiotics, my micro-review suggests strongly that B Infantis is the most promising candidate of all the strains.
Sanjay Patole and others in Perth have performed a number of meta-analyses of the clinical trials of probiotics in the preterm, and the most recent focuses on the trials that have used B Infantis, as either the sole probiotic, or as a component of a mixed probiotic preparation. (Batta VK, et al. Bifidobacterium infantis as a probiotic in preterm infants: a systematic review and meta-analysis. Pediatr Res. 2023).
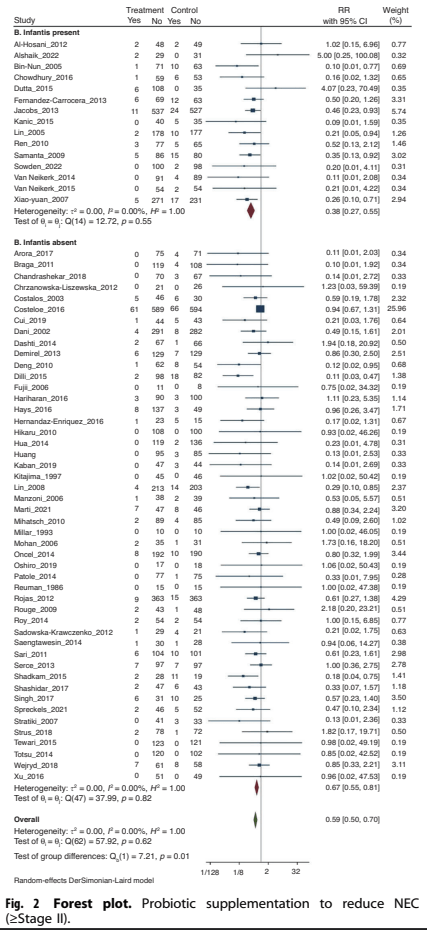
As you can see from this Forest plot, there are a large number of trials, including B Infantis or without, with a total of over 14,000 babies. The trials which included a B Infantis in the treatment group had a reduction in NEC with the RR of 0.38 (0.27, 0.55 95% CI) compared to those with other organisms which had an RR of 0.59 (0.50, 0.70). The statistical test for subgroup differences suggest that this differential impact is unlikely to be due to random effects.
That SR also includes similar plots for overall mortality, preparations with B Infantis RR=0.65 (0.48, 0.88) compared to placebo, preparations without B Infantis compared to placebo, RR= 0.78 (0.67, 0.91). For Late-Onset Sepsis, RR=0.8 (0.63, 1.01) with B Infantis, compared to 0.86 (0.77, 0.97) without B Infantis.
The minor problem with this SR is that, as mentioned B Infantis is a subspecies of B Longum, the other subspecies being B Longum ssp Longum. A few RCTs have stated that they used B Longum, without specifying the subspecies, at least one of them used a mixture “Restore” that they report as including B Longum, when I went on the website of the company that produces Restore, they state that it is a B Longum ssp Infantis. However, the study had so few cases of NEC, 2 vs 1, (and is so badly written that I cannot tell whether group A or group B received the probiotics!) that it would make no difference to the meta-analysis. Another small trial used a mixture containing B Longum, but neither the publication nor the website of the company states which subspecies is in the mixture “Darolac”.
Indeed, this is a major problem in many parts of the world, the quality control standards and certainty of the identification of the strains in the various available products are often very poor. Mixtures may contain no live organisms, different organisms to those claimed, and/or pathogens. It is essential to find a preparation with the production standards required to ensure that you are really giving the organisms you want, and not others.
If B infantis, or other “probiotic” organisms, are able to enter the blood stream, which usually occurs only when the intestinal barrier has been breached, they do not produce lipopolysaccharide endotoxins, as do most pathogenic Gram negatives, which are responsible for much of the inflammation. Nor do they produce any exotoxins, as does Group B streptococcus and some Gram negatives. Which is why most of the babies described in the literature have had minor illness when they have a bacteraemia with these organisms, they just are not very pathogenic. It really is essential to make sure that you are not giving any of the bad bugs when you try and supplement with the good guys.
The most likely candidate, as a single strain of organism that could vigorously colonise the preterm intestine, digest HMOs, decrease inflammation, decrease intestinal permeability, inhibit the growth of pathogens, and has been shown to be a preferentially effective probiotic against NEC in this species selective Systematic Review is B Infantis. The very organism that the FDA has just forced off the market.
UPDATE: of note, David Mills, a real expert in this subject, sent a comment (and a reference) pointing out that many commercial probiotic preparations, that are supposed to contain B Infantis often do not! There may be other Bifidobacteria, such as a B Infantis that turned out to be B lactis, and there is even variation from lot to lot. This casts a shadow over the meta-analysis above, as it is possible that some of the preparations in the B Infantis group may not actually have contained B Infantis, clearly, all future studies must reliably ascertain the strain used.









Keith, thanks for the post. One item to consider when looking retrospectively at probiotic trials is the accuracy in naming of the bacterial species involved. In particular, B. longum subsp. infantis has a long history of being misnamed/typed in numerous probiotic products (and in academic research papers). These problems are mostly gone with the advent of each/cheap whole genome sequencing but historically researchers just assumed probiotic companies had the right species designation. If you follow the link below this is not always true. B. longum subsp. infantis was designated a subspecies in 2008 and there are still errors in its nomenclature (again in both products and science papers). Thus I consider all meta-analyses like this where the authors assume the species designation is correct….with a grain of salt. See the link below.
Clearly a key part of any probiotic clinical trial should be repeated genomic analysis to ensure the probiotic you have is what you want.
Dave Mills
UC Davis
https://www.nature.com/articles/pr2015244
Thank you Keith. You have done a very good job researching the details of different probiotic organisms and especially the function and benefits of B infantis. I have been sceptical about “probiotics” being good for infants because it is like saying antibiotics are good without defining which antibiotic and dose are good for which infection. As you say there are many different probiotics used with different numbers of bacteria when we know that many of the different formulations do not contain what the manufacturers claim. Now we need to know whether a combination of probiotics is needed and whether that improves their action. We also need strong control of the manufacture and composition of different formulae.