The latest trial to fail to find a benefit of erythropoietin (Epo) for neonatal brain protection has just been published (Wu YW, et al. Trial of Erythropoietin for Hypoxic-Ischemic Encephalopathy in Newborns. N Engl J Med. 2022;387(2):148-59). The HEAL trial, a multicentre randomized controlled trial in 500 asphyxiated term infants undergoing cooling found a similar incidence of death, of neurologic impairment, and of developmental delay at 2 to 3 years with Epo or placebo. There was also no difference in MRI injury, either as a percentage or when looking at the patterns of injury, or in discharge neurological examination.
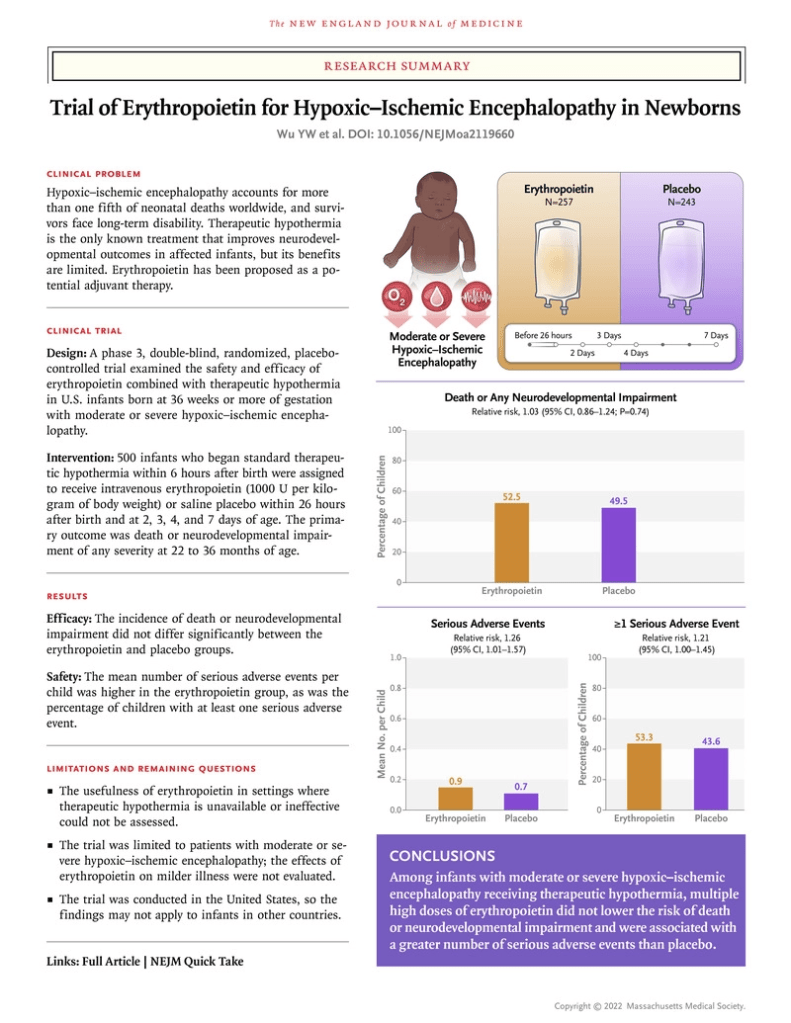
Epo was started before 26 hours of age, in fact at a mean of just less than 18 hours, the dose was 1000 u/kg for a total of 5 doses, about 1/4 had severe (Sarnat 3) encephalopathy, the reminder being moderate.
Erythropoietin is effective in many animal models of HIE, so why did it not work in this trial? At first I thought that maybe it was given too late, but the authors suggest that maybe it was given too early! They put it this way “Other possible explanations for our negative findings include toxic effects of erythropoietin administration early in the injury cascade when combined with therapeutic hypothermia; suboptimal dosage or timing of administration, because later doses may be most effective; and differences in injury mechanisms between preclinical models of hypoxic–ischemic encephalopathy and human hypoxic–ischemic encephalopathy” In the first of the 2 animal studies they refer to in support of their statement about timing, in rats with cerebral artery occlusion the Epo was given starting a week after the injury, and improved outcomes, in the other, in mice with an MCA occlusion, Epo was given starting 3 days after injury and improved outcomes. But I haven’t seen anything to suggest that Epo only works if given late, but not if given early (I am by no means an expert in this literature). Indeed the lamb studies of Alistair Gunn show efficacy of Epo given starting 30 minutes after the injury. His studies, however, suggest that there is no additional benefit if given in combination with therapeutic hypothermia (Wassink G, et al. Recombinant erythropoietin does not augment hypothermic white matter protection after global cerebral ischaemia in near-term fetal sheep. Brain Commun. 2021;3(3):fcab172). Indeed there may be adverse effects of the combination (Dhillon SK, et al. Adverse neural effects of delayed, intermittent treatment with rEPO after asphyxia in preterm fetal sheep. J Physiol. 2021;599(14):3593-609).
It remains possible that if you don’t have access to therapeutic hypothermia, that Epo may have a role, and a much smaller RCT from northern India, (n=100) did suggest a benefit among non-cooled infants. (Malla RR, et al. Erythropoietin monotherapy in perinatal asphyxia with moderate to severe encephalopathy: a randomized placebo-controlled trial. J Perinatol. 2017).
The authors report that the overall incidence of serious adverse events was higher in the Epo group than the controls, but the definition of what is an SAE compared to an adverse event is rather subjective, so hypertension, is considered an SAE, whereas convulsions (which were a bit less common with Epo) are considered a non-serious AE, if you exchange those definitions then there is no “significant” increase in SAE. I know you should not redefine things after seeing the results, but lumping all the very different adverse events which they consider serious together seems to be already quite questionable. Individually, there is nothing which looks different to random variation. Which is to say that Epo is probably safe, but also very likely ineffective when used like this for this indication.
There are other trials, or at least 1 other trial in Australia (PAEAN), which seems to have completed enrolment but has not yet reported outcomes, so it is possible that this will change, but it seems unlikely as there isn’t a hint of an advantage of Epo in the HEAL trial. The PAEAN trial also is in babies under hypothermia, and also plans to randomize prior to 24 hours of age.
The outcomes of babies with HIE after hypothermia remain problematic, there are many infants with long term motor or intellectual difficulties, and further research will be essential. What should be the next trials? There are of course a few already being performed, one promising therapy being studied in a multicentre European trial is allopurinol, in the ALBINO trial, with a sample size of over 800 it will be the largest trial yet of HIE; large trials are needed for this condition, as the chance of seeing an impact as great as the impact of hypothermia are small, but moderate improvements in outcomes, needing large sample sizes to show them with certainty, could be very important. There are a couple of small trials of melatonin, which suggest a possible impact, and a current multicentre trial in Italy has a sample size of only 100, which is underpowered for all except dramatic effects.
This figure from a 2015 review article suggested some other possibilities
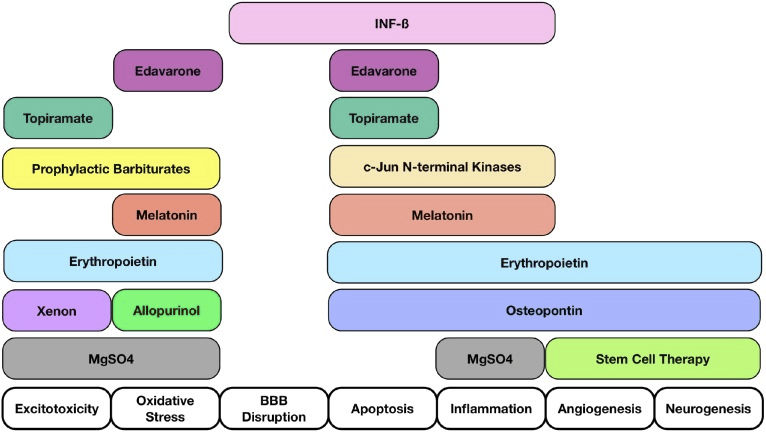
The most easily investigated of those possibilities are the same ones that I wrote about 10 years ago! I still think that prophylactic high-dose phenobarbitone is worth investigating, with the same rationale, a small RCT pre the cooling era showed benefit, there are theoretical and some pre-clinical data suggesting a role also.
But for now, there is no proven therapy that improves outcomes beyond the benefits, important and real but limited, of therapeutic hypothermia.









To understand the full picture all trials should report the rate of fetal asphyxia and stillbirths in the population studied. Ian Grant used to say that the quality of obstetric care could be measured by the frequency of neonatal seizures. There is no better neuro-protection than good obstetric care.
I understand what you are saying, but to truly know the denominator would be difficult! The population data wouldn’t help to know if Erythropoietin was effective, for an individually randomized trial. I think the population data would be more important for observational or epidemiologic studies.
Even with the best Obstetrics, cases still occur, with uterine rupture, massive abruptions, cord prolapse, unpredictable cases of shoulder dystocia and so on. In our practice there are not that many that are clearly avoidable, although there are sometimes things that could be improved. Prevention is always the best medicine, but some things can’t be prevented.
Another good review. I especially like that you allude to the possibility that therapeutic hypothermia alone has the major benefit, with no additional benefit of the adjunctive therapy Epo. I just hope that readers of the manuscript don’t withhold Epo in patients that do not qualify for hypothermia (prematurity, arrival at center after 6 hours) when there is a hint of efficacy in several studies. I don’t think we can ethically study Epo as a primary/solitary treatment for HIE when the data suggests benefit of hypothermia, but maybe a larger multicenter trial can be done with Epo vs supportive care in babies who don’t qualify for hypothermia based on accepted criteria.
I think that is true, we do need more studies of Epo when cooling is not available. It is one of the reasons that I was critical of the suggestion in the HEAL trial that Epo increased adverse effects, as I don’t think the data really support that interpretation, and it might inhibit further trials if it is taken as true. Currently the trials suggest that, in the absence of cooling, Epo is at least not harmful, even if we cannot be sure that it is helpful.