Or perhaps “probably better than the current fashionable alternative” might be a better title. Phenobarbitone (or phenobarbital, I will call it PHE) is one of the oldest anticonvulsants out there, and because of little good data, remains the drug of first choice for seizures in the newborn. We know, however, that it often doesn’t work very well, many infants continue to have clinical seizures after being loaded with appropriate doses, and even more continue to have electrical seizures.
Seizures are bad for your brain. Every article I read about this has some sort of qualified statements, such as “accumulating evidence suggests that…” or “there are indications that perhaps…” or as in this new study “there is mounting evidence that seizures themselves are harmful”. Is there any doubt? Having a good proportion of your neurones firing synchronously for no good reason other than to make you move clonically, or smack your lips, or even just to increase your cerebral oxygen consumption without clinical signs, is surely damaging. Of course the underlying disorder is the most important factor in outcomes, but the same disorder without seizures is always better than with them. Unless you have no neurones left to fire!
So let us take it as self-evident that newborn babies with asphyxia, or meningitis, or stroke, would be better off if they had fewer seizures, all other things being equal. Is there a medication that can reliably reduce seizure burden? If so we could then investigate what the impacts are on long term outcomes. I hope it is evident that a medication that reduces seizures will not necessarily improve outcomes despite all that I have said above, if there are other adverse impacts. We need to find the best medication that reduces seizures while improving long term outcomes.
What medications are effective in neonatal seizures? There are not many good trials, and those that exist are old, many without EEG monitoring as a routine. The older trials of PHE vs Phenytoin showed little difference between the two drugs, which begged the question whether they were both equally effective, or equally ineffective?
Newer agents hold the promise of being less toxic and possibly having beneficial impacts in the long term. The one which, by common acclaim of neonatal neurologists, has become the go-to anticonvulsant after PHE is levetiracetam, which I have difficulty pronouncing so I will call it LEV.
A few years ago we were starting to use topiramate as the second-line agent, and then, almost overnight LEV became the drug everyone was talking about, and it was introduced into our protocol as the second-line agent for infants continuing to have seizures after being fully loaded with phenobarbitone. Why LEV rather than the alternatives? I have to admit that it was fashion, rather than any compelling evidence! The article I am discussing today admits almost as much, that the evidence base for LEV use in the newborn was entirely from case series. Nevertheless, there was so much hype about the potential benefits of LEV compared to PHE that the new RCT was designed to randomize more babies to LEV than to standard, phenobarbitone, therapy. Unfortunately, that design reduces the power of the study; for the same total number of babies in the trial, power is less if the numbers are substantially unequal.
(Sharpe C, et al. Levetiracetam Versus Phenobarbital for Neonatal Seizures: A Randomized Controlled Trial. Pediatrics. 2020) The NEOLEV2 trial.
Despite this primary reservation, the trial is an extremely important landmark, being a comparison of PHE with something else as first-line treatment of neonatal seizures. Bravo to the study group, this was a much-needed trial.
Full-term infants with continuous video EEG monitoring were entered if they had EEG confirmed seizures, I was unsure how they would do such a trial at first, in most places there is intermittent review of the EEG trace by various individuals, and sometimes the occurrence of seizures is not recognized until the next morning, for this trial they used a commercial service that “continuously” reviewed the traces. I’m not sure how this works exactly, how can you continuously review several EEG traces? Apparently, the commercial service employs EEG technicians at a distance to watch the wavy lines in real time as they are produced by the monitor. They mention the use of seizure detection software, but such software has low PPV and imperfect sensitivity in the newborn, so it was used to assist in seizure detection, rather than a diagnostic tool.
The babies had many different diagnoses, more than 50% were post-asphyxia, some had strokes or infections and a smattering of other diagnoses. EEG monitoring continued for 2 to 6 days after the trial started.
When electrical seizures were confirmed the babies were randomized. Here again I am a little uncertain, the article uses the plural ‘seizures’: how many seizures were required? Were infants randomized after a single brief seizure, or only after a series, and was there a minimum?
I know that most babies with HIE (54% of the study infants) have multiple seizures, and indeed most babies that we diagnose as having seizures for any reason have multiple episodes, but if you are ‘continuously’ reviewing the traces what happened when the techs saw the first seizure? Did they wait until there were a few more before calling the centre? There are some more details in an article they authors published about the EEG monitoring system (Sharpe C, et al. Assessing the Feasibility of Providing a Real-Time Response to Seizures Detected With Continuous Long-Term Neonatal Electroencephalography Monitoring. J Clin Neurophysiol. 2019;36(1):9-13). But that doesn’t answer some of my questions. They do discuss the automated seizure detection software, and confirm that there are many false positives, the big problem with the Persyst software is that it isn’t a specifically neonatal algorithm, and neonatal seizures are different, apparently, in an electrophysiologic sense.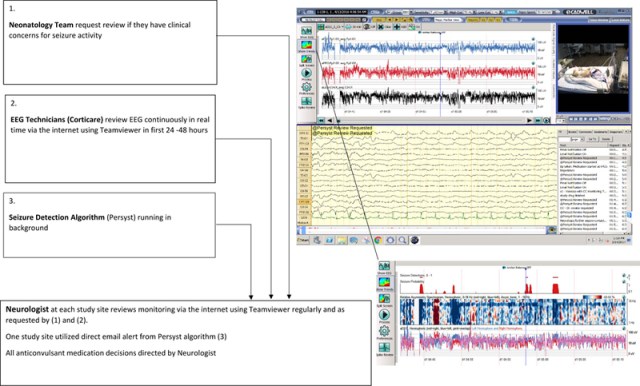
One of the findings of the study underlines the difficulties involved, there were in the end 106 babies randomized (64 LEV, 42 PHE) but 12 of them were excluded after EEG review as they were not finally thought to have seizures prior to the medication being given. Unfortunately, this was more common in the smaller PHE group, with 9 being eliminated, and, with other issues making evaluation of the primary endpoint impossible in 11 other babies (8 LEV and 3 PHE), the final sample size was modest n=83 (53 LEV, 30 PHE).
The primary outcome variable for the study was complete elimination of seizures for a 24 hour period, the outcome was originally meant to be 48 hours but the authors clearly describe in the methods why and when it was changed.
Phenobarbitone was more effective. As you can see in the following figure, the initial load stopped seizures in 70% with PHE and only 21% with LEV. Giving the second dose (by protocol if seizures not controlled) added a few more in each group, so prior to switching to the other treatment (again, done by protocol) 24/30 PHE babies had shown efficacy compared to only 15/53 LEV babies. Even after the switch phenobarbitone seems more effective (though the numbers start to be quite small).
LEV was safer, with less cardiovascular or respiratory depression.
The results also don’t say how long it took to eliminate seizures, it is well known that clinical seizures tend to stop well before electrical seizures, which often continue for 24 hours after PHE is administered. I don’t know if the same thing happens with LEV.
What next? Long term follow up of the NEOLEV2 babies will be important, it would certainly be surprising if the LEV babies had better outcomes, but we need to know the size of any difference between groups.
Although there are concerns about PHE and long term impacts, PHE also has cerebral protection effects. In older children, much higher doses of PHE alone have been used to control refractory status epilepticus, without apparent damaging effects. If you remember the trial by Hall et al from 1998, asphyxiated infants who had not yet had seizures were randomized to 40 mg/kg of PHE or placebo, and they had better outcomes at 3 years of age. Hall RT, et al. High-dose phenobarbital therapy in term newborn infants with severe perinatal asphyxia: a randomized, prospective study with three-year follow-up. J Pediatr. 1998;132(2):345-8). But PHE wasn’t very good at preventing seizures in that study! The Cochrane review of barbiturates for perinatal asphyxia points out the limitations of the evidence, and the poor quality of the outcome data, both in that study and in the little other data available.
Given the potential benefit of PHE for long term outcomes revealed in those trials, and the advantage of PHE for seizure control in NEOLEV2, I think the next trial should have one arm with PHE dosing increasing beyond even 40 mg/kg. The authors of NEOLEV2 suggest that higher LEV doses should be investigated, but they don’t present any data about anticonvulsant efficacy and serum concentrations in this publication. It may be that LEV just isn’t very good for neonatal seizures, and pushing the doses higher won’t necessarily improve efficacy.
We certainly need larger trials, and trials large enough to examine effects between diagnostic subgroups, or perhaps which just enroll HIE, or HIE and stroke babies. The infrastructure put in place in San Diego and Auckland for this trial is interesting, and could potentially be enlarged, but the randomization of significant numbers of babies who were finally thought not to have had seizures is a problem. The authors note that some of the EEG techs reviewing the traces did not have much neonatal experience. Also interesting is that the EEG traces were all reviewed by 2 neurophysiologists to determine if the drug worked, in case of discrepant decisions a 3rd neurophysiologist reviewed the traces in order to tie-break. Such a review was required 22 times.
Over 1/4 of the time, experienced neonatal neurophysiologists couldn’t agree between themselves whether a baby’s seizures had stopped or not! Makes me feel better about having difficulty with the traces sometimes.










Thank you for your supportive assessment of our publication. We certainly appreciate the interest and value on-going discussion, so have addressed some of your points.
In response to the question on duration of seizure, in our study we treated any electrographic seizure detected (10 sec minimum by definition) and our cohort included two infants with less than 30 seconds of cumulative electrographic seizure burden before treatment.
With respect to the time taken to eliminate seizures, infusions of the study drug were given over 15 minutes and a further 15 minutes was allowed for the treatment to take effect. If seizures persisted after that 30-minute period, or recurred at some later point, treatment escalated. This meant that the initial 40mg/kg load of LEV was completed a minimum of 45 minutes before escalation to PHB.
Finally to clarify our inter-reader agreement – You note that a 3rd reviewer was required as tie-breaker 22 times in reviewing the EEG records. However, we needed agreement in EEG on 2 or 3 decisions per record to confirm:
· there had been seizures prior to treatment
· that there was correct escalation of drug treatment (if that occurred)
· and that seizures did or did not terminate with drug treatment
So for the 106 infants the rate of disagreement between our reviewing neurophysiologists was circa 8% rather than a quarter of the time.
Best regards
Dr Cia Sharpe on behalf of the NEOLEV2 investigators
Thank you for the extra information. This is such difficult research to do, I congratulate your team for doing this study. We struggle with seizure surveillance in our NICU, and I know we are not alone; babies with very frequent seizures or frankly in status are sometimes not recognized until the day staff come into the NICU. We need to do better! Trials like this will help a lot.
Thanks also for the clarification about the reviewing neuophysiologist agreement.