A new single centre RCT of permissive hypotension (PH) compared to “standard treatment” (ST) of very preterm infants 24 to <30 weeks GA, with a mean BP lower than their GA has just appeared (Alderliesten T, et al. Treatment of Hypotension of Prematurity: a randomised trial. Arch Dis Child Fetal Neonatal Ed. 2025). In the intervention, PH, group, infants only received treatment if they developed signs of poor perfusion. In the ST group they immediately received a fluid bolus and a dopamine infusion, followed by adding dobutamine, then epinephrine, hydrocortisone was given if the baby needed more than just dopamine. They don’t explicitly say what the goals of the cardiovascular support were, and what triggered increases in dose or the addition of other agents. I assume that the goal was to have a mean BP above the GA, but perhaps the goal was the GA+3 or something, it should have been stated. About 1/3 of the 40 ST babies only had a fluid bolus, and no further catecholamine support, about 1/4 of the 46 PH babies eventually had a fluid bolus. From table 2 it looks like there were 19 babies who had a fluid bolus and went on to receive catecholamines in the ST group, and 6 PH babies, at least, if the numbers in the table were the maximum doses received.
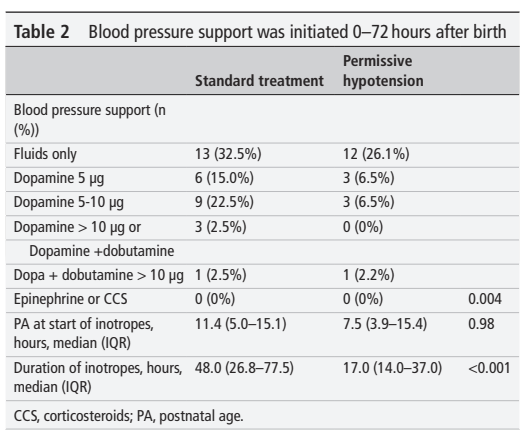
The table is poorly explained, the floating numbers in the right-most column are presumably p-values, and I assume that the first of them (0.004) refers to the whole block of data about what seems to be maximum intervention received, and not to the 0 vs 0! It seems that only 32 of the 40 ST babies actually received any treatment for their hypotension.
The babies were enrolled into the study between 2011 and 2018, and the study was eventually terminated, in part because of falling eligibility. This period probably covers the introduction of routine delayed cord clamping in preterm deliveries, which has led to a major reduction in the diagnosis and treatment of hypotension.
The interventions in the PH group presumably are for 15 babies who developed signs of poor perfusion, or a mean BP of GA-5 mmHg (according the discussion section they were mostly for low BP); but 12 of them had good perfusion after a fluid bolus, and they had no further intervention. Why there are, it seems, 8 ST babies who didn’t receive any intervention isn’t clear. What you can also see in this publication, is that the mean BP in the 2 groups was just about identical, despite the interventions in the ST group.

The primary outcome of the trial was developmental outcome using Bayley version 3 cognitive and motor scores at 24 months corrected age. Secondary outcomes included mortality and the usual NICU complications.
The major finding of the trial is that the 2 groups had almost identical long term outcomes. The mean scores, and the proportion <-1SD below the mean, and traditional combined outcomes, were all very similar between the groups, as were the acute complications, NEC and IVH. The mortality was slightly higher in the permissive hypotension group 6/46, vs 3/40, but they note that there were 2 PH babies who died after the intervention period because of LOS with G-negative organisms and shock.
I do not understand the presentation of the cognitive and motor scores; they are identical between the 2 study arms, but they are presented as being approximately 91 in the 2 groups. But, further down in the main results table, they are presented as “using age at BSID-III assessment corrected (CA) for prematurity” and the means are closer to 101. Surely the primary outcome was already the BSID scores corrected for prematurity?
The interventions, therefore were different to our HIP trial (Dempsey EM, et al. Hypotension in Preterm Infants (HIP) randomised trial. Arch Dis Child Fetal Neonatal Ed. 2021;106(4):398-403). In our trial the infants in both groups were also free of signs of poor perfusion, and they all received a fluid bolus, then the intervention group were started on 5 microg/kg/min of dopamine, compared to placebo in the controls. This meant that the BPs were different between groups:

We have recently published our long term outcomes (Marlow N, et al. Outcomes of extremely preterm infants who participated in a randomised trial of dopamine for treatment of hypotension (the HIP trial) at 2 years corrected age. Arch Dis Child Fetal Neonatal Ed. 2025) which showed no major differences, but with the proviso that we were very underpowered, and terminated the trial well before the planned sample size.

There was, if anything, a trend towards a better outcome in the intervention group.
In the new trial, one can really quibble about the interventions used. Although dopamine is most commonly used for this indication around the world, which is why we used it in the HIP trial, it acts as a pure vasoconstrictor in the preterm infant, improved perfusion of any organ has not been shown with dopamine use in the newborn. In particular dopamine is a cerebral vasoconstrictor, and has been shown in several models to decrease brain blood flow, or brain oxygenation.
Second line therapy with dobutamine is also debatable, dobutamine is a vasodilator, and does not reliably increase BP, so using it to treat hypotension is questionable (although it may be effective in improving perfusion).
There are a few concerns with this article, the delay between completion of follow up (2020) and publication is very long, the specific items that I have mentioned above, but most importantly they didn’t reference the original article which invented the term “permissive hypotension”! Dempsey EM, et al. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F241-4.
The sad fact is that we still don’t know what to do about the baby with reasonable clinical perfusion who has a numerically low BP. The totality of the current data suggests that it is entirely acceptable to just wait and see, especially in the current era of delayed cord clamping, where hypovolaemia is very unlikely.








