We have known for a very long time that bilirubin can cause an encephalopathy leading to acute and chronic clinical impacts, the most severe chronic impacts being kernicterus, a disabling movement disorder and nerve deafness. What seems to be the case is that bilirubin bound to albumin doesn’t cross the blood-brain barrier, and that the BBB, which appears to develop early, and be functional by 20 weeks, is easily disrupted in the newborn. Bilirubin staining of the basal ganglia has been shown on autopsy specimens at much lower concentrations than are associated with kernicterus in term babies. This finding led to the performance of the large multicentre NICHD network phototherapy trial, which is very limited by the lack of a funky acronym. Can I suggest that we start calling it the “LITEPOP” trial: Longterm ImpacTs of Early Phototherapy for Outcomes of Preterms?
There have been multiple studies looking at “free” or “unbound” bilirubin and its impacts, which are hindered to some extent in difficulties measuring it. The subject of this post is a new re-analysis of data from that NICHD early phototherapy LITEPOP trial, which finished enrolling in 2005, and finished follow up 2 years later, with the primary publication (Morris BH, et al. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N Engl J Med. 2008;359(18):1885-96) appearing in 2008. If you remember, or if you have never heard about this, the primary outcome was “death or NDI” (groan…). There were nearly 2000 babies of 501 to 1000 g bwt randomized at 12 to 36 hours of age if they had never had phototherapy. The intervention was to have phototherapy started immediately and continued until it fell below 85 micromol/L (5 in the US units). The control group had phototherapy to keep the bilirubin either below 137 (500-750 g bwt) or below 170 (751-1000 g).
The results are a very apposite demonstration of the problems with such a composite outcome. Mortality was just about equal in the two groups, 209 vs 201 died before discharge, and a further 17 vs 21 died after discharge, or 24 vs 23% total mortality.
The NDI data were presented as among the entire groups (survivors tested plus deaths), i.e. 235/902 vs 275/902, which is statistically significant, 26% vs 30%, (RR 0.86 (95% CI 0.74–0.99)) but because of the 1% higher mortality in the early phototherapy group, the combined outcome of “NDI or death” was not considered significant.
If we recalculate the outcome of “NDI” just among the survivors who were tested, 235/672 vs 275/684, 35% vs 40%, and the proportion with low PDI scores 127/672 vs 152/684, 19% vs 22%, or with severe hearing loss, 9 infants vs 28, or with athetosis, 2 infants vs 10, then the differences all suggest benefit of early phototherapy.
Babies in this study had a blood sample stored at 5 days of age, which was later measured at one of 2 laboratories. Although they used the same method, (the peroxidase method) and the same analyser, there were enough differences between the 2 labs that the results were converted to z-scores for each lab, and are reported in the newest publication as percentiles of the z-score.
The first publication reporting these data, however, was in 2010, Oh W, et al. Influence of clinical status on the association between plasma total and unbound bilirubin and death or adverse neurodevelopmental outcomes in extremely low birth weight infants. Acta Paediatr. 2010;99(5):673-8. The reporting of the free or Unbound Bilirubin (UB) in that first publication was weird. The graphs appear to show UB concentrations in the microg/dl range,
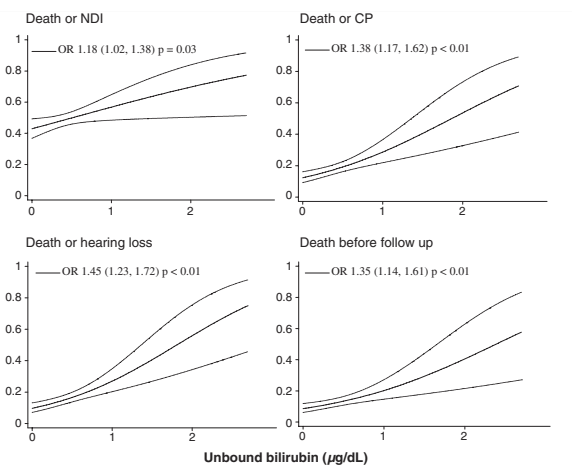
But a correction published 3 years later reveals that they actually show the UB divided by 0.3 “we have standardized the unbound bilirubin by dividing the actual values by the standard deviations which is 0.3. The standardized values (in units) were used in the presentation of data in this figures which appear to be 0–3.0 units. The actual unbound bilirubin values are 0–0.9 mcg/dL”. I don’t understand how this “standardized” the data, it was clearly just a screw up that none of the 89 authors noticed. (I exaggerate).
You may also remember a secondary analysis published 4 years after the original manuscript Tyson JE, et al. Does aggressive phototherapy increase mortality while decreasing profound impairment among the smallest and sickest newborns? J Perinatol. 2012;32(9):677-84. In that publication they performed a subgroup analysis dividing the smaller babies into those ventilated and those who were not. Whether this was a preplanned subgroup or not is unclear; the publication states rather vaguely that they had plans “to relate the risks and benefits of AgPT to baseline risk factors including measures of severity of illness”. In that secondary analysis of a subgroup, using Bayesian analysis, they claimed to show that it was highly likely that phototherapy increased mortality among ventilated infants of 500-750 g. That analysis also shows that there was a probable decrease in mortality among non-ventilated 500-750 g babies (13% vs 25%), a probable decrease among larger babies who were ventilated (16% vs 19%), and a possible increase among larger non-ventilated babies (8% vs 6%). My interpretation of which is that the differences are very likely just random variations between groups. However, the authors of that secondary analysis were much more emphatic that phototherapy was decreasing the anti-oxidant benefits of bilirubin, and was generating oxidant injury and was increasing mortality in the smallest babies, if they were ventilated.
So why have they come back to these data now? (Arnold CC, et al. Unbound bilirubin and risk of severe neurodevelopmental impairment in extremely low birthweight newborns. Pediatr Res. 2025) The authors state that there are new concerns about lipid emulsions leading to increases in Free Fatty Acids and displacing bilirubin from albumin, leading to an increase in UB. But such concerns are not new at all, they date back to the 90’s or further. Perhaps more relevant is that a new method for measuring UB has become available, which might make it more clinically useful, and there are new statistical methods available, including the TMLE (Targeted Maximum Likelihood Estimation), which apparently uses machine learning to give conservative explanations of correlations between variables.
For this analysis they concentrated on “severe NDI” or sNDI, which was “based on the NDI outcomes with the strongest associations with the phototherapy regimen in the NICHD aggressive vs conservative phototherapy” LITEPOP RCT which was : a score on the Bayley II Mental or Psychomotor Developmental Index of 50 or less or a level of 5 for gross motor function (GMF), or needing bilateral hearing aids.
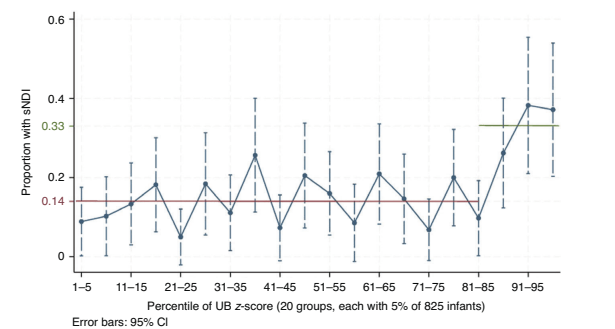
They divided UB into 20 slices, based on percentile of UB, as you can see from the figure below, there seems to be a threshold effect, with sNDI increased substantially for the top 15% of UB concentrations on day 5 of life.
There was very poor correlation between the total bilirubin and UB concentrations, as you can see below. But almost all of the UB concentrations associated with worse outcomes were above the threshold for phototherapy in the early treatment group, only 1 or 2 of the dots are in the upper left quadrant. You can also see, what I was not aware of previously, that far more than half of the dots are above 5 mg/dl, which implies that the phototherapy was not very effective at keeping the total bilirubin below the treatment threshold.

Most of the babies with “sNDI” did not have classic clinical signs of kernicterus, choreo-athetoid cerebral palsy, oculomotor problems, and high-tone nerve deafness. This could be because bilirubin encephalopathy is not so specific in the affected regions in very preterm infants, especially in the presence of acidosis, sepsis etc.
What should we do about this? If UB or “free” bilirubin measurement becomes more widely available, then the lack of correlation with total bilirubin strongly suggests that UB should become the variable of choice for monitoring hyperbilirubinemia in full term infants with jaundice. We could perhaps decrease the proportion of babies who get intervention while improving the prevention of kernicterus, which continues to affect somewhere around 1 in 100,000 babies, and while rare is devastating. The potential benefits among preterm infants are numerically greater, if a new trial (the UNBEATEN trial, UNbound Bilirubin EArly TreatmENt trial, I claim copyright) could show that screening for, and treating, to maintain UB < the 85%ile, improved neurological and developmental outcomes.
At the same time the trial could investigate whether the possible impact of phototherapy on mortality among the subgroup of ventilated extremely preterm infants in the NICHD trial was a real phenomenon or not.









As regular consumers of Dr. Barrington’s Neonatal Research blog, we were happy to see that he had devoted the blog of February 2, 2025 (Bilirubin is Bad for the Brain! Who Knew?) to issues addressed in our recent publication.(1) Included in the blog were comments regarding previous secondary analyses of the NICHD Neonatal Network Aggressive vs. Conservative Phototherapy trial(2) in which we reported a possible mortality risk associated with exposure to phototherapy. These were conservative Bayesian analyses,(3,4) that identified a 99% probability that aggressive phototherapy increased mortality, a 97% probability that it reduced impairment, and a 99% probability that it reduced profound impairment in ventilated infants <750 g BW.(5)
Dr. Barrington interprets these findings as likely due to random variation among different subgroups. He could be correct. That said, the possibility that phototherapy may increase the mortality of small, preterm babies is also supported by evidence from the only large trial in which infants were randomized to receive phototherapy or no phototherapy (the Collaborative Phototherapy Trial which enrolled infants in the 1970s).(6) The results were compatible with a 49% increase in death in ELBW infants in the phototherapy group (relative risk = 1.49 [95% confidence interval 0.93-2.40]). This finding was largely ignored apparently because the p value exceeded 0.05. However, conservative Bayesian analyses identified a 92% probability that phototherapy had increased the mortality of ELBW infants in that trial.(3)
Were they not born early, extremely preterm infants would have normally developed in darkness in utero for several months or longer before birth. The long-standing assumption that sustained exposure to bright light during phototherapy is innocuous with no serious adverse effects for even the most immature, translucent, and vulnerable babies is neither evidence-based nor biologically plausible. There is some evidence that phototherapy may increase the risk of developing cancer(7) or as Dr. Barrington has noted, the risk of epilepsy. More importantly, phototherapy could directly or indirectly increase the morbidity or mortality of small preterm infants by any of a variety of different mechanisms.(8,9)
In assessing how phototherapy might be best administered, the NICHD Neonatal Research Network is conducting a randomized trial of conventional continuous phototherapy versus cycled phototherapy for babies <750 g birthweight or <27 weeks gestation. Cycled phototherapy can be used to substantially reduce total phototherapy exposure with only a minimal increase in total serum bilirubin.(10) The goal of the current Network trial is to determine whether use of cycled phototherapy can decrease the predischarge mortality of these infants below that with continuous phototherapy with an equal or lower risk of neurodevelopmental impairment at 2 years adjusted age. As of March 5th, 2025, the neonatal component of this trial is nearing completion with only 81 more babies needed to reach the total of 1700 infants to be enrolled.
Bilirubin neurotoxicity is believed to result from entry of unbound bilirubin into the brain.
As Dr. Barrington explained, unbound bilirubin Z scores greater than the 85th percentile for the infants in the Network trial of aggressive vs. conservative phototherapy were associated with a substantially increased risk of total neurodevelopmental impairment.(1) Due to the limitations inherent in these observational analyses, the true unbound bilirubin threshold that causes impairment might be lower than the observed 85th percentile. In any case, bilirubin neurotoxicity may adversely affect the development of an appreciable proportion of small preterm infants.
If accurate measurements of unbound bilirubin become widely available as may occur in the foreseeable future,(11) they are likely to supplant total serum bilirubin measurements in assessing the risk of bilirubin neurotoxicity in the neonatal period. This would be an important advance that would facilitate more individualized treatment decisions to reduce unnecessary exposure to phototherapy. Whether continuous or cycled phototherapy is used in the future, we certainly agree with Dr. Barrington that a new large, randomized trial would be needed to establish evidence-based treatment thresholds for unbound bilirubin in small preterm infants. The challenge is to find the optimal balance between preventing bilirubin-induced neurotoxicity while minimizing the potential adverse effects of any treatment methods used to prevent such toxicity. This challenge clearly requires thoughtful reconsideration of long-held practices and a commitment to rigorously examine both the benefits and hazards of our interventions.
Jon Tyson, MS, MPH
Cody Arnold, MD, MSc
Claudia Pedroza, PhD
References:
1 Arnold C, Maric I, Wong RJ, Tyson JE, Stevenson DK. Unbound bilirubin and risk of severe neurodevelop-mental impairment in extremely low birthweight newborns. Pediatr Res 2025; Jan 23. doi: 10.1038/s41390-025-03872-x. Online ahead of print.
2 Morris BH, Oh W, Tyson JE, et al. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N Engl J Med. 2008;359(18):1885-96.
3 Wijeysundera DN, Austin PC, Hux JE, Beattie WS, Laupacis A. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol. 2009;62(1):13-21.e5.
4 Quintana M, Viele K, Lewis RJ. Bayesian analysis: Using prior information to interpret the results of clinical trials. JAMA 2017; 318:1605-6.
5 Tyson JE, Pedroza C, Langer J, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Does aggressive phototherapy increase mortality while decreasing profound impairment among the smallest and sickest newborns? J Perinatol 2012;32(9):677-84.
6 Scheidt PC, Graubard BI, Nelson KB, et al. Intelligence at six years in relation to neonatal bilirubin levels: follow-up of the National Institute of Child Health and Human Development Clinical Trial of Phototherapy. Pediatrics. 1991;87(6):797-805
7 Tyson JE, Miller C. Whether neonatal phototherapy increases the risk of cancer in children is a disturbing unresolved issue. Evid Based Med 2017, 22:39-40.
8 Stevenson DK, Wong RJ, Arnold CC, Pedroza C, Tyson JE. Phototherapy and the risk of photo-oxidative injury in extremely low birth weight infants. Clin Perinatol. 2016; 43:291-5
9 Jasprova, J, Dal Ben M. Hurny D, et al. Neuro-inflammatory effects of photodegradation products of bilirubin. Sci Rep 2018;8: 7444.doi: 10.1038/s41598-018-25684-2
10 Arnold C, Tyson JE, Pedroza C, et al. Cycled phototherapy dose-finding study for extremely low birth-weight infants. A randomized clinical trial. JAMA Pediatr 2020;174: 1-8.
11 Hegyi T, Kleinfeld A, Huber A, et al. Unbound bilirubin measurements by a novel probe in preterm infants. J. Mater. Fetal Neonatal Med 2019; 32: 2721–6.
I certainly don’t think we should ignore the difference in mortality in the subgroup of the smallest babies on ventilators; but there was no difference at all in overall mortality, which means that there was a balancing lower mortality among larger intubated babies, and smaller non-ventilated babies. It is hard to think of a mechanism which would increase mortality in one group, and decrease it in another.
It is interesting what you say about the Collaborative Phototherapy trial, because I cannot find any published data showing a difference in mortality between groups in that trial. The only one of the publications that mentions mortality (Scheidt et al Pediatrics April 1990) states that the mortality in the phototherapy group was 72 compared to a mortality of 62 in the controls, and doesn’t break them down into weight categories. Are the figures you quote published somewhere? I think it would be very important to have them easily available. Hopefully the new trial, nearing completion that you mention will give us some important new data on which to base future intervention.
It is a stretch to infer that the Network trial showed that the aggressive and conservative phototherapy have an equal effect on the risk of death for all ELBW infants. While the RR was 0.97, the 95% credible interval was wide (0.80 -1.19). As judged from the study findings, the probability that aggressive phototherapy reduces overall mortality was 60%. However, that means that there was still a 40% probability that it increases mortality. Moreover, even if uninterrupted exposure to bright light has no effect on the mortality of all ELBW infants, it could increase the mortality of < 750 g infants, particularly those who are ventilator treated, if the translucency of their skin and tissues, immaturity, illness, or other plausible effect modifiers increase the risk/benefit ratio of phototherapy. With all that is at stake for these babies, this concern and the worrisome findings of the only two large trials have prompted the current Network trial in the hope of reducing their mortality.
The data from the Collaborative Phototherapy Trial are available through Pub Med as published by Lipsitz PJ, Gartner LM, Bryla DA.. Neonatal and infant mortality in relation to phototherapy, Pediatrics 1985; 75: 422–426 and as noted in a chapter by Jeffreys Maisels in a textbook by Sinclair JC, Bracken MB (Effective Care of the Newborn Infant. Oxford University Press: Oxford, UK, 1992, Table 47, p 532).
Jon Tyson, MS, MPH
Cody Arnold, MD, MSc
Claudia Pedroza, PhD