Many of us were already aware of the general direction of the results of this trial, a multi-centre RCT of surfactant with or without budesonide (Manley BJ, et al. Intratracheal Budesonide Mixed With Surfactant for Extremely Preterm Infants. JAMA. 2024), disappointingly negative results, with no impact on survival or on any index of lung injury.

In order to put this in context, I have just been trying to search the literature for all the prior trials, knowing that there were 2 trials from Yeh’s group in Taiwan, the 2nd being a very positive trial performed in 3 centres, 2 in Tawian and 1 in Chicago. There have been several systematic reviews published, the most recent of which was published in a journal called “Respiratory Medicine and Research” and dates from June this year. In that recent SR I saw many more trials than I was aware of, and I must say, this foray was extremely disturbing. There is an enormous amount of very questionable research being published and/or referenced. Below is the Forest plot which includes both studies of early budesonide inhalation, as well as studies of budesonide instillation with surfactant. Bassler 2015 is of course, the NEUROSIS trial, which showed slightly higher mortality with budesonide inhalation, but less BPD among survivors. This Forest plot shows BPD among survivors from all the early inhaled/instilled steroid trials.

I tried to find some of the other trials. Heo2020 is actually a retrospective chart review, with no consent, and no mention of randomization. Wen2016 is from “World Latest Medicne Information” (sic) and impossible to find. Among other studies included in the SR were Cao2018, published in 2018 in the “Journal of Pediatric Pharmacy” which, as far as I can tell, stopped production in 2017, Zhou2019 was also apparently published in the same ex-journal in 2019. Kou2019 is also untraceable, with the link in the reference list of the SR leading to a completely different article.
Some of the real articles in the SR include Pan2017 (Pan J, et al. [Clinical efficacy of pulmonary surfactant combined with budesonide for preventing bronchopulmonary dysplasia in very low birth weight infants]. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19(2):137-41, the abstract is in English, for the rest I used Google translate) 15 babies were randomized to budesonide with their surfactant, and 15 to surfactant alone. The incidence of BPD (defined as needing oxygen at 28 days) was 1 vs 6, which the authors claim was “significant and statistically significant”; of course, it is far from being statistically significant, by Fisher Exact, p= 0.08. Ping2019 also seems to exist, it appears to be a report of a prospective RCT, and seems to show a reduction in moderate to severe BPD, which is undefined in the abstract, which is all I can get hold of. Ke2016 is a 4 group RCT, with 46 subjects per group, one of the groups being budesonide plus surfactant, and one being surfactant alone, the others being budesonide by inhalation using 2 different methods. Yao2021 was published in “American Journal of Translation Research” and purports to be a trial using nebulized budesonide.
It is impossible to know how much of this research was actually ever performed, how much is fictional, how much was invented by AI, how much is re-quoting of numbers from other reviews without adequate checking of the original.
This is very disturbing to anyone wanting to provide high-quality medical care, based on reliable evidence, to sick babies. One way to deal with all this is that, when we write or review a Systematic Review for publication in a reliable journal, we must insist on only prospectively registered trials, with publicly available data. We will have to perform more independent IPD meta-analyses, where the review authors share, and can examine each others anonymised individual data, if we are to be able to rely on the best evidence for our patients.
The background, therefore, to PLUSS, was of a single high-quality trial of moderately large size (n=265 total), Yeh 2016, which doesn’t even appear in the Forest plot above (probably because they did not publish the incidence of BPD among survivors) but which showed a substantial decrease in oxygen needs at 36 weeks, from 50 to 29 %, which was due to a reduction in both moderate BPD (31 to 20%) and severe BPD, (19 to 9%). The RR for “BPD or death” was therefore 0.63, 95% CI 0.5 to 0.8.
If we recalculate the primary outcome of PLUSS as a relative risk of the primary outcome, it was 0.96, with 95% CI of 0.9 to 1.03. In other words, not only was there likely no benefit, with the residual possibility of a small benefit or a minor harm, but no overlap with the CI of the previous trial either. Also of note, there was no impact of budesonide in any of the prespecified subgroups either. Only a post hoc subgroup analysis of the infants with an FiO2 >50% at treatment showed some hint of a benefit.
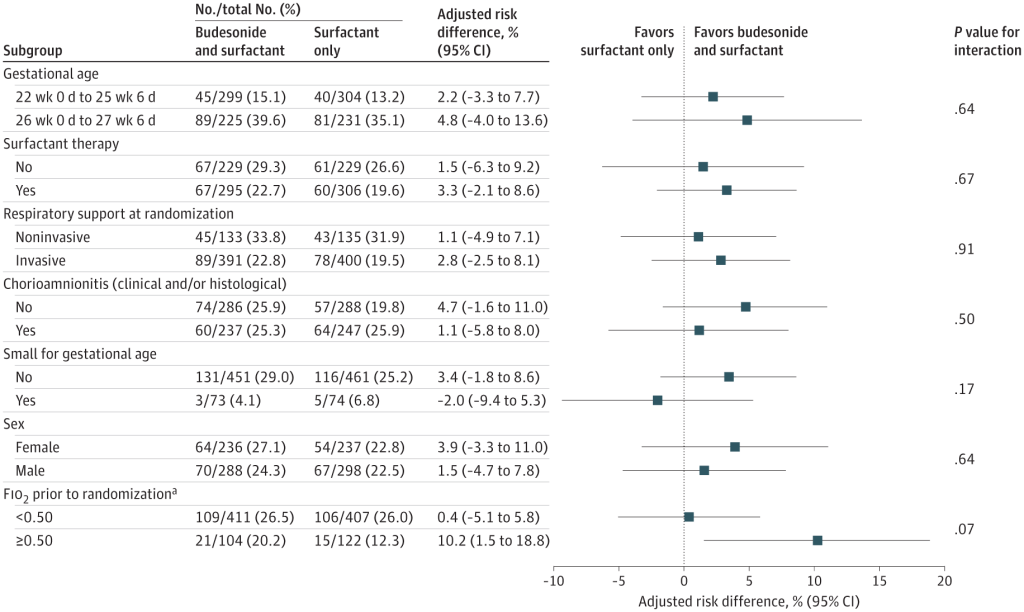
So why is PLUSS so different to Yeh et al? PLUSS used a different surfactant (poractant rather than beractant in the previous study) had more immature infants, (average GA 25.6 weeks compared to 26.5 weeks), who needed less oxygen on average at intervention (average of 30%, compared to a mean FiO2 in Yeh et al of 60%) and had a maximum of 2 doses of budesonide, compared to a maximum of 6 doses in Yeh’s study.
Yeh had lower mortality, and less BPD among survivors than PLUSS, in the control groups. Mortality in the controls was 16% in Yeh’s trial, and 20% in PLUSS, while BPD in survivors was 55% vs 72%.
In PLUSS, infants who had already received one dose of surfactant were eligible to be randomized at the time of the second dose, if they were still < 48 hours of age. In fact about half of the infants (in both groups) had already received a dose of surfactant without budesonide prior to being randomized. But one of the pre-planned subgroup analyses was restricted to infants getting their first dose of surfactant, and that also showed no benefit of budesonide, as you can see from the figure above, “Surfactant therapy” means previous surfactant without budesonide prior to randomization. So the ‘No’ results are for babies getting their first dose, and there is an almost identical primary outcome (Death or BPD) among these babies. Because prior surfactant treatment was permitted in PLUSS, the average age of administration of the study drug was later, at 5 hours, compared to 2 hours in Yeh et al.
The new study was much larger than Yeh, and allowed surfactant via LISA/MIST, compared to all the babies being intubated in the previous trial. The differences in outcome, with this new high-quality trial showing no benefit with budesonide, could be because of the differences I have discussed, or some other currently uncertain effect, or simply because of random variation. As I already mentioned, the confidence intervals of the primary outcome of PLUSS do include the possibility of a small benefit of budesonide instillation, but do not overlap with the CI of the trial of Yeh.
Post hoc subgroup analyses, such as the one performed here of above and below 50% O2, should always be treated with great suspicion. Introducing new, unplanned analyses after the data have accumulated is very risky, and inflates the possibility of a type 1 error, suggesting a difference which is not real. In addition, the interaction term was not significant, which means that, although the subgroups with FiO2 above and below 50% had different results, there is not a statistically significant difference between those subgroups. For all these reasons, budesonide instillation with early surfactant cannot be recommended based on this trial, even for babies with very high oxygen requirements. It may be that another trial enrolling only infants with very high oxygen requirements could possibly show a benefit, but that is a possibility that can only be answered by doing such a trial.
The BIB trial, currently underway in the USA does not, however, restrict enrolment to infants with a high FiO2, and is in many ways similar to PLUSS, with the main difference being a restriction to the first dose of surfactant, and the inclusion of infants up to 28 weeks and 6 days gestation. BIB has completed enrolment, and, you never know, perhaps we will see some results at PAS next spring. Once we have those results, we should be able to make an evidence-based decision about routine budesonide instillation with surfactant, and we will be able to ignore all the very small, potentially unreliable or perhaps fictitious trials in the current Systematic Reviews. Perhaps, if someone has the time and the funding, we could do an IPD meta-analysis, and only those investigators who are able to supply the individual patient data, from pre-registered trials, could collaborate to give the best possible information for future babies, and for future trials.









Keith, I applaud you for calling out the Junk Science. With all the pay-to-play predatory journals, anything can get published these days. It is high time to establish some rules about what should and what should not be included in a meta-analysis. MAA has been held to represent the highest level of evidence, but that is no longer true today…..
Martin Keszler MD
mkeszler@wihri.org
Brown University
Pingback: Neonatal Research Shorts : October 2025 | Neonatal Research