The question in the title was recently adjudicated in US courtrooms, with an enormous award of damages to the family of a premature infant who developed Necrotising Enterocolitis, and survived, but has, apparently, major neurodevelopmental issues. In July, the jury awarded the infant’s mother $95 million in compensatory damages and another $400 million in punitive damages against Abbott who produce the preterm formula that the baby had received. A previous case against Mead-Johnson (the only other preterm formula producing company in the US) was also decided against that company in favour of a family whose baby died of NEC, they were awarded $60 million. As far as I can tell, the basis for the awards is that the companies failed to warn the families of the risks of their formulas. I don’t know how the companies are supposed to warn the families, or how neonatal programs across the USA are responding to this situation, but there are apparently hundreds of other lawsuits that have been, or are in the process of being, launched.
I just did a Google search for Necrotizing Enterocolitis, lawsuit, and breast milk, 15 of the first 16 results were web pages of US law firms touting for business, some of them being hidden as pretend legal news sites. The sites rapidly open active chat windows, trying to get you to communicate with them to find out how much money you could win. The other one was a real US news station.
No company could possibly run the risk that each baby who receives their formula and then develops NEC will cost them millions of dollars. There is a real chance that the 2 companies that currently make artificial formula for preterm infants will pull out of the market, and what then?
There are also apparently parents in NICUs (in the USA) currently who are refusing preterm formula, as they have heard about these lawsuits, or have heard from lawyers that preterm formulas are dangerous, and cause NEC. Fortunately, this has not yet arrived in Canada, but such things have a tendency to osmotically pass through the semi-permeable membrane which is the longest border in the world separating us from the madness.
Without the preterm formulas, when feeding preterms with artificial formula we would have to return to supplementing term formula with additional protein, calories (probably mostly fat) and minerals (especially phosphorus, but also calcium and others). With no reason to think that this would be preferable in terms of NEC risk, indeed almost certainly this would not reduce NEC, but would introduce other risks: of errors, of contamination, or of excessive osmolarity.
The courts are clearly not the best place to determine scientific causality. So what does the science say? There are no RCTs comparing mother’s own milk (MoM) to artificial formula, for reasons which I hope are obvious, nor are there any trials comparing MoM to donor human milk (DHM) for the same reasons.
There appear to be 12 RCTs which have compared artificial formulas, all of those tested being based on cows’ milk, to DHM for preterm infants. As the most recent Cochrane review notes, there are multiple differences between the trials
- Four trials compared feeding with term formula versus unfortified donor breast milk (Raiha 1976; Davies 1977; Schultz 1980; Gross 1983). In all of these trials, term formula or donor breast milk was the sole diet.
- Eight trials compared feeding with preterm formula versus donor breast milk, either as the sole diet (Tyson 1983; Lucas 1984a; Cristofalo 2013), or as a supplement to maternal breast milk (Lucas 1984b; Schanler 2005; Corpeleijn 2016; O’Connor 2016; Costa 2018).
- The trials varied according to type of donor breast milk, and whether donor breast milk feeds were nutrient‐fortified or not.
- Five trials used donor breast milk collected from mothers who had delivered an infant at term (Raiha 1976; Davies 1977; Schultz 1980; Lucas 1984a; Lucas 1984b). Two of these trials used ‘drip’ breast milk (Lucas 1984a; Lucas 1984b). One trial used preterm donor breast milk (Schanler 2005), one trial used both term and preterm donor milk (Gross 1983), and five trials did not specify the type of donor breast milk (Tyson 1983; Cristofalo 2013; Corpeleijn 2016; O’Connor 2016; Costa 2018).
- In all trials except Tyson 1983, the donor breast milk was pasteurised.
- Four trials used donor breast milk with multinutrient fortifier added empirically or as indicated (Schanler 2005; Cristofalo 2013; Corpeleijn 2016; O’Connor 2016). Cristofalo 2013 used human milk‐based fortifier, and the other trials used cow’s milk‐based fortifier.
The 4 oldest trials, published 1976 to 1983 used standard term formula, and only one of them (Gross 1983) reported NEC, which was nearly 5 times more frequent with term formula than with DHM! However, the trial was small and the numbers with NEC were tiny; so the difference in incidence of NEC, 3/26 babies receiving term formula compared to only 1/41 who got DHM, is not statistically significant (RR 4.73 95%CI 0.52-43.09). I don’t think statistical significance matters to law courts, however, so maybe that finding could be used in court to show that term formula is associated with the same risks of NEC as preterm formula.
In some ways, this is a philosophical question of the meaning of causation. NEC is more common in very preterm babies receiving artificial formula than in those receiving MoM, with an intermediate risk with DHM. Does that mean that MoM is protective, or does this mean that DHM causes NEC? And that formula is even more dangerous?
Or does it mean that the pathogenesis of NEC is very complex, that there are some components of human milk that are protective, and that there is an advantage of MoM over DHM. The advantage of MoM over DHM is, I think, in part because it is not pasteurized, and perhaps also because there are some differences in the composition of the milk of mothers who deliver preterm. Also, babies getting MoM are more likely to get maternal colostrum, which is not available from milk banks, and may be especially protective, perhaps maternal colostrum is part of the explanation why babies receiving MoM have lower NEC rates than DHM.
Preterm milk composition differences from term milk, which is the majority of what is supplied by almost all milk banks in the world, include higher concentrations of protein and some minerals. There is also probably some impact of PT birth on the oligosaccharide composition of milk (Human Milk Oligosaccharides HMOs). Bifidobacteria and Lactobacilli are present in most unpasteurized human milk, and are able to metabolise HMOs, which humans are not able to digest. Preterm milk has higher concentrations of HMOs, but they apparently are less diverse. I have previously posted about the potential role of DSLNT Di-Sialyl-Lacto-N-Tetraose (a specifically human HMO), which has now been shown in 4 studies to be present in lower concentrations in the breast milk of mothers whose babies develop NEC despite receiving MoM, Autran, Hassinger, Masi, Van Niekerk. It also protects against NEC in Lars Bode’s rat model. Apparently it is difficult to synthesize, but hopefully we will eventually get trials to see if NEC can be prevented by adding DSLNT to MoM, DHM or preterm formula.
This graphic is from a recent Chinese study (Zhang W, et al. Causative role of mast cell and mast cell-regulatory function of disialyllacto-N-tetraose in necrotizing enterocolitis. Int Immunopharmacol. 2021;96:107597), which examined the role of DSLNT in a rat model of NEC, which reproduced the protective effect in rats. It also showed that human intestinal specimens from NEC resections demonstrated Mast Cell activation compared to specimens without NEC, and also that an enzyme (chymase) produced by Mast Cells caused epithelial loss; and that in a human foreskin cell model DSLNT protected against this. Which is apparently a new potential mechanism for the beneficial effects of DSLNT.

In the current climate I cannot imagine any business putting money into trying to develop a preterm formula with DSLNT to try to reduce NEC risks, however. I suppose it will be up to public funding agencies to do so, and then hopefully for public organisations to profit, if it can be shown to be protective.
For the present, all very preterm babies at increased risk of NEC should have access to DHM when mother is unable or does not wish to provide enough MoM, which means all babies born at less than 33 weeks. From 33 weeks onward, the risk of NEC appears to be, currently, very close to the baseline risk in term babies, although very good recent data are difficult to find.
All of the discussion in this post has made some very questionable assumptions, which include (1. that NEC can be diagnosed reliably, and (2. that NEC is a single disease in the very preterm. I know that neither point #1, nor point #2 are actually reliably true. But I also know that NEC can be a devastating disease, with a high mortality and long term consequences, despite these considerations.
NEC in full term infants, including those with cardiac malformations, post-asphyxia, and after gastroschisis repair, is probably even more pathophysiologically variable, and not clearly impacted by breast milk use. Among late preterm infants the protective impact of breast milk is also unclear, they have been excluded from most trials. One of the earliest trials to show a benefit of breast milk in prevention of NEC, by Lucas and Cole, included babies of under 1850g birth weight, some of whom were over 32 weeks. They had 4 cases of NEC among 34 to 36 week infants who only received formula, compared to 0 in the breast milk groups. One very interesting finding in that early study was that babies who received breast milk AND formula had the same risk of NEC as those who received 100% human milk. Here is the table of results from that study including all gestational age groups:
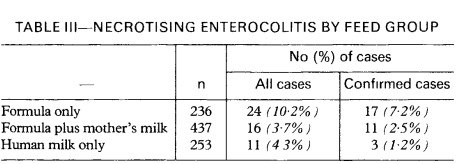
Which, if I was a lawyer/expert witness, I would quote as good evidence that it is not formula that causes NEC, but breast milk that is protective.









Hi Keith
I liked very much this last post of yours- Let me tell you that you are doing a great , enormous job! Congratulations indeed ! hold on , thanks!
best wishes
Paolo Manzoni , Italy
Keith, future studies in NEC need to consider the amount of antibiotic exposure in those premature infants
Thanks for these comments though I was surprised to see that there was no mention of the role of probiotic (especially multi-strain) organisms on protection from NEC. We began routine supplementation with multiorganism formulations added to all feeds whether MOM, DBM, or preterm formula many years ago and in concert with the move towards predominant BM feeds, experienced marked reduction in the incidence of NEC and , in particular, nearly the disappearance of surgical NEC. Mike Maurer,MD
I whole-heartedly support the routine use of probiotics in at-risk infants. Specifically with regard to the issues in this post, fresh breast milk contains bifidobacteria and lactobacilli, not contained in DHM post-pasteurization, or in formula. I didn’t get into that in detail in this post, but I hope my views, and my understanding of the literature have been clear in many other posts! The current regulatory situation in the USA is really concerning, and the impact of FDA rulings has been to increase risks for huge numbers of babies.