The Exclusive Human Milk Diet sounds superficially immediately convincing: “We should only give milk products derived from human milk to human babies”.
But, is it more than just a catchy phrase? The major company which is responsible for producing a human milk based breast milk fortifier is heavily into promoting its use. They are certainly responsible for substantial profits for their shareholders. Unfortunately, many of the publications about the EHMD have been marked by important conflicts of interest, and, most importantly, they have not performed the most important relevant comparison. Which is, if you only use human milk (mother’s or donor) for every feed of the very preterm baby, is there an difference in clinically important outcomes between a multi-component fortifier derived from donor human milk, and one derived from bovine milk?
What does the evidence currently show?
Mother’s own milk, unpasteurized, is almost certainly associated with the lowest rate of NEC compared to other sources of milk. If there is insufficient mother’s milk, then pasteurized donor milk is the next best choice, leading to lower rates of NEC than artificial formula. I think this is now well-enough established, despite the limitations in the data, that I won’t even put the references in.
When it comes to breast milk fortification, is there any evidence to support the use of human milk derived fortifier compared to bovine milk derived fortifier, among infants who only receive human milk feeds?
There are only 2 relevant trials, one I have discussed previously, more than once, from Toronto, O’Connor et al, which randomized infants, who were all receiving only human milk feeds, to a bovine fortifier or a human milk derived fortifier, and found no effect on NEC or other clinically important outcomes.
The other is a Swedish trial which is now available as a preprint. Jensen GB Effect Of Human Milk-Based Fortification in Extremely Preterm Infants Fed Exclusively with Breast Milk: A Randomised Controlled Trial. Available at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4529245. This is the not yet peer-reviewed report of the results of a trial for which the protocol has previously been published. (Jensen GB, et al. Nordic study on human milk fortification in extremely preterm infants: a randomised controlled trial-the N-forte trial. BMJ Open. 2021;11(11):e053400).
In this new trial 229 infants <28 weeks GA were randomized before they reached 100 mL/kg/d of oral milk feeding. They all received mother’s milk or donor human milk, and the milk fortification was individualized, with standard fortification plus extra protein or fat as required, all of them being from human milk in the intervention group, or cow’s milk in the controls. The cow’s milk fortifier used was not standard between the 7 NICUs involved, but they continued to use whatever they were previously using. The study babies were not allowed to receive formula in either group prior to 34 weeks PMA.
The primary outcome was a composite of death, NEC, and late-onset sepsis, prior to discharge. All x-rays were independently analysed, and outcomes were assigned by investigators masked to group participation.
There was no difference in the primary outcome, nor in any component of that composite, in particular there was no difference in Necrotising Enterocolitis.

You can see the results below:
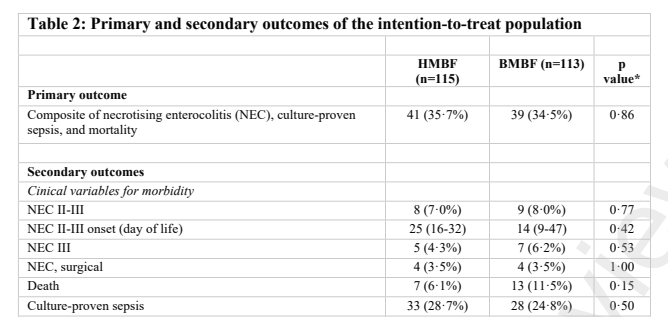
If I do a quick and dirty meta-analysis of the 2 trials, assuming there are no other studies that I am unaware of (yes, it does happen!), we can see the following outcomes, using a random effects model, and the RevMan version 5 software:
Necrotising Enterocolitis, Bell stage 2 or 3
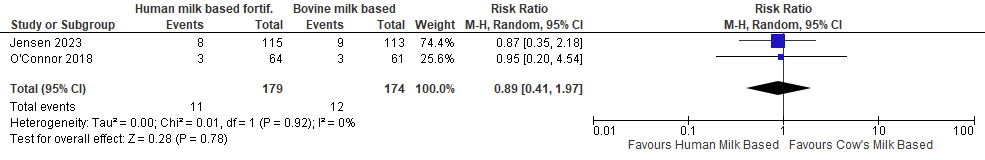
Mortality
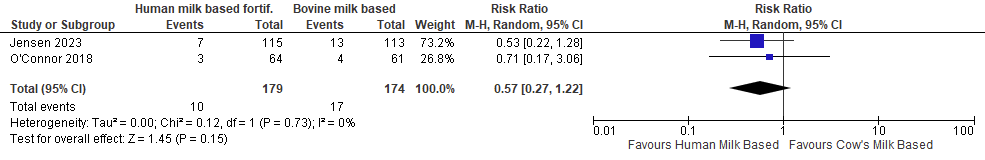
Late-onset Sepsis
There is no clear difference in any outcome between the different sources of breast milk fortifier.
A recent article claimed to show that human milk derived fortifier was cost-effective. The costs of the human milk derived fortifier, among the 7 hospitals involved, were between about 250,000 and 1.6 million annually, and they claim that there was an overall cost saving associated with the EHMD (of supposedly up to 3.4 million dollars a year, a number which is being touted by the major producer in their publicity). However, that publication was based on no presented data, just what participants in a round-table discussion said to each other; I really don’t understand how it got published.
In fact if you do an internet search you may well see the headline “Prolacta fortifiers save hospitals up to 34M annually”! Clicking on the link will make it clear that is up to 3.4 million (not 34), but nowhere is it clear that the cost savings are only if you compare using Prolacta products to using formula as supplement to mother’s milk.
I think it is, indeed, likely that an EHMD diet might save money if you compare it to using artificial formula, but that is not a choice which any of us would now make. As Human milk derived fortifier is very expensive, at somewhere over $10,000 US per baby, and there is no clear advantage at all over bovine milk derived fortifier, then it can’t possibly be cost effective in comparison to using a cow’s milk based fortifier.
There are several review articles, often, again, marked by conflicts of interest, which are sometimes not clearly declared, which suggest that it is important to avoid bovine milk proteins, to reduce NEC. That assertion cannot be supported by the literature. There are many differences between human milk as currently used (either fresh mother’s milk, or pasteurized donor milk) and artificial formula; the extensive processing of bovine milk to create sterile formula, and the lack of Human Milk Oligosaccharides in the product, the lack of human immunoglobulins, and many other differences may be much more important than the source of the protein. Preterm babies are immunologically incompetent; there is no primary reason to suppose that artificial formulas increase NEC because of the source of the protein, it may well be because of some of the many other differences between formula and human milk.
Another issue is that breast milk has become a saleable commodity in some places, the New York Times has an interesting article discussing some of the implications of this, one of which is that poorer mothers could possibly end up selling their milk instead of giving it to their own babies. In most places outside of the USA breast milk donation is an altruistic act of lactating mothers, to whom we should all be immensely grateful.
To be strictly evidence-based, as I always strive to be, the current evidence (as you can see in the Forest plots above) continues to have wide confidence limits, and is therefore consistent with either a substantial increase or decrease in NEC with human fortifier compared to bovine. If the producers of human milk based breast milk fortifier want to continue to promote it as a way of reducing NEC, compared to a human milk diet fortified with bovine fortifier, then they should be forced to perform an adequately powered trial.
Even a 25% reduction in NEC would be of major clinical benefit, and a reduction from 8% to 6% might be cost effective too. It would also be within the confidence limits of the currently available data. Such a reduction could be sought with a RCT with a sample size of about 2,500 per group. If that was prohibitive, and I don’t think it should be given the enormous profits possible if they could prove efficacy for reducing NEC, then focusing on a higher risk group, such as the <26 week infant, and hypothesizing a larger decrease such as reduction of 40%, could give a more manageable sample size. A reduction from 10% to 6% would need a sample size of about 700 per group, with a power of 80% and an alpha of <0.05.
As the evidence currently stands, I consider the myth of the Exclusive Human Milk Diet : BUSTED.









Thanks Keith for this very important post, we were waiting for the result of this study from Sweden… What do you think would be the impact of these findings on the practice, especially in the units where the use of “EHMF” became part of the routine in the nutrition of preterm infants?
I think that there is no justification for the additional costs of human milk based fortifier over bovine milk based fortifier. Artificial formula should be banned in at-risk babies, but once you are using Mom’s or donor milk, there is no evidence that one fortifier is preferable over another.
I much prefer the powdered fortifier, a there is very little volume displacement when fortifying, so the babies receive more human (hopefully Mom’s) milk. But that is a personal preference not supported by any literature, as there is no literature comparing powdered to liquid fortifiers, to my knowledge.
Keith: You suggest banning formula but when my babies fail to grow on fortified donor milk rather than super fortifying donor milk I alternate 24 cal formula with donor milk. I suspect the risk of nec to be the same but I tend to see better growth. You still want to ban formula?
Jeffrey Pietz
jpietz1@valleychildrens .org
I often fortify to 26 or 28 kcal per ounce. And I admit the data are not strong for doing that rather than adding some formula. I think the best approach, if growth is inadequate on 24 cal milk, is probably to analyze the milk, and individually add either more protein or more calories. I can’t currently do that so we just add more fortifier. Is that better than introducing some formula, idk. but I don’t see that adding 24 cal formula is likely to help much, maybe some concentrated 26 cal formula.
If your breast milk is low octane say 12kcal/ounce it will take a lot of fortifier to get 24 or 26 kcal. This is why alternating the donor milk with 24 kcal formula achieves better growth. With EPF 24 you know how many calories you are giving. Often going from donor fortified to”30” kcal to EBM “24” alternating with 24 EPF gives my kids a sudden improvement in growth. After all we are not very good at assessing caloric content in the NICU. Enfamil does that better.
Keith: You suggest banning formula but when my babies fail to grow on fortified donor milk rather than super fortifying donor milk I alternate 24 cal formula with donor milk. I suspect the risk of nec to be the same but I tend to see better growth. You still want to ban formula?
Jeffrey Pietz
jpietz1@valleychildrens .org.
Great article, thank you Keith.
Don’t think it quite meets the strict entry criteria for your mini M/A but there was another small RCT (Uthaya S et al., 2022)[1] of n=38 babies born <30 weeks gestation and feeding on their own mothers’ milk. They were randomised to receive for any shortfall either a human milk-derived preterm formula + human milk-derived fortifier or standard (bovine milk-derived) preterm formula + bovine milk-derived fortifier. The intervention lasted until 34 weeks postmenstrual age. The primary outcome of interest was MRI-assessed body composition at term equivalent, and there were no significant differences between groups. There were also no significant differences for the secondary clinical outcomes of NEC and sepsis.
Microbiome and clinical outcomes have more recently been reported for a larger cohort (n=126) of babies recruited to the same study (Embleton NJ et al., 2023).[2] Interesting (perhaps surprising?) finding by the authors was that there was no overall effect on gut microbiome richness or diversity, and they also reported no significant differences between groups in any of the main clinical outcomes, including NEC, sepsis, mortality. (See Table 2).
One notable outcome reported in this paper was of an infant who developed late-onset vitamin K deficiency bleeding (VKDB) on day 73 (luckily while still in the NICU). This infant was in the exclusive human milk + human milk fortifier group. We have discussed previously this propensity to later VKDB with exclusive breast feeding [and/or an exclusive human milk diet] if there is no provision of sufficient ongoing dietary vitamin K supplementation.[3] Expensive, human milk only diet milks and human milk-derived fortifiers may, if not properly supplemented, therefore potentially place babies at risk of important micronutrient deficiencies.
[1] https://www.sciencedirect.com/science/article/pii/S0378378222000822
[2] https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2801833
[3] https://www.jthjournal.org/article/S1538-7836(22)18363-7/fulltext
Thanks for the comment, Paul. I was aware of this fascinating study, but I left it out of the quick MA because there was formula use in the control group. I think they only reported surgical NEC also. As I mentioned, I think that the standard of care now, despite some limitations in the data, is to give only mother’s milk, or human donor milk to babies at risk of NEC. The Embleton study surprisingly showed no clear impact of any of the interventions on the microbiome. I just saw some fascinating information about the impact of emulsifiers on the microbiome. As noted I am sceptical that the source of the protein is the problem with formula, this new (to me) data suggests that Carrageenan, Polysorbates and other components may have serious impacts on the intestinal microbiome.
Finding more effective ways to improve the dysbiosis of the preterm infant should be a priority.
I feel the Canadian study alluded as myth buster evidence had certain concerns. One is was the trial sample size large to detect a difference? The trend generally suggested an EHM diet had favorable results.
We need to be careful demonizing private corporation.
If we want to reduce costs and/or improve care- we will need to accept private corporations are important and be receptive to be part of their sponsored trials.
Private corporations played large roles in the neonatal development of inhaled nitric oxide and surfactant .
There is a tiny grain of truth in what you are saying, BUT: Private corporations played almost no role in the development of either nitric oxide or surfactant.
I was one of the first people in the world to treat a neonatal animal (piglets) with NO, with no funding from private corporations, and none of my studies had any private funding. None of the initial trials (from Roberts, or from Kinsella) were funded by private corporations, and the main RCTs were funded by public money. In a few, such as NiNOS, the NO was eventually supplied free by the company, but that was their only contribution. The only inhaled NO trial that was entirely funded by private money was the poorest study, it wasn’t a bad study, just not as powerful as the other major trials.
All of the development of exogenous surfactant as a therapy was by committed individuals with public funding, starting with the recognition by Mel Avery and Jere Mead that surfactant deficiency was crucial in HMD. CuroSurf is so named because of Todd CUrstedt and Bengt RObertson, who spent years finding the best way to extract and stabilise surfactant, using public money. Colin Morley who developed a powdered form of surfactant, and Fujiwara, who was the first to show a clinical benefit of exogenous surfactant, all did their studies using public money.
I struggle to find any example of an improvement in neonatal care that has been initiated, or funded in a major part, by private corporations. Private corporations have reaped the benefits of research funded by public agencies, however. With NO being the best example, iNOtherapeutics made millions, not because they supported good research, but because they happened to have a legal agreement with Warren Zapol and Harvard, that allowed them to be the sole beneficiaries, for years, of the publicly funded research that showed that nitric oxide was EDRF, that you could administer NO mixed with inhaled gases, and that inhaled NO selectively reduced PVR. NONE of that was funded by private corporations.
The grain of truth in what you say is that the trials showing that Human-Milk based fortifiers are ineffective are underpowered. If Prolacta, MedoLac, and any other corporation want to make money from the “exclusive human milk diet” then they must fund adequately powered trials to prove it.
Pingback: NEC awareness day, 17 May 2025. What is new in NEC prevention? | Neonatal Research