A major revision of the Cochrane review of inhaled nitric oxide gas in the preterm infant is now available. (Barrington KJ, Finer N, Pennaforte T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane database of systematic reviews (Online). 2017;1:CD000509). It includes 2 new publications, a small RCT from China, and the large multicenter RCT in preterm babies still requiring respiratory support at 5 to 14 days: the NEWNO trial (listed in the review as Yoder 2013). This was an unusual situation for me as the NEWNO trial has still not been published; we waited a while to finalize the review as we thought that it would be better that NEWNO was in print before including it in the meta-analysis. Eventually, because we had access to much of the summary data, we decided, with the delays in publication of the primary data for the entire study, that we should proceed. It is possible of course that we may need to make some minor adjustments to the review once the peer-reviewed publication appears, but it is unlikely, I think, that there will be any major changes to make. The main conclusions are very likely to stand.
One of the great things about Cochrane reviews is that they are (supposed to be) kept up to date. With frequent new literature searches and inclusion of new trials as they appear. Which means that authoring a review is a serious commitment. Also this time for the new reviews there are a number of innovations in the Cochrane Collaboration standards since the last time I updated the review. As well as the graphics to show the risk of bias evaluations, each new review (or updated review) will have a Summary of Findings Table, using Grade standards (and the Grade groups on-line software).
In case you haven’t seen them before here is one of the risk of bias figures: it should be clear that this is really what is meant, not that there has been bias, but that a lack of allocation concealment (for example) increases the risk of bias. For this review, many of the trials were funded by industry (previously not a common occurrence in neonatology) so we decided to add the column entitled ‘Funding Source’. There are abundant data to show that industry funding skews the medical literature, I therefore think we should have such a column for all Cochrane reviews, but we must be clear that although this increases the risk of bias, it doesn’t necessarily mean that those trials marked in red were actually biased. By most standards, in fact, the industry-funded trials in this review appear to have been free of the problems with many such trials, like inappropriate composite short-term outcomes, controls groups almost designed to have poorer outcomes, selective publication and selective reporting of outcomes.
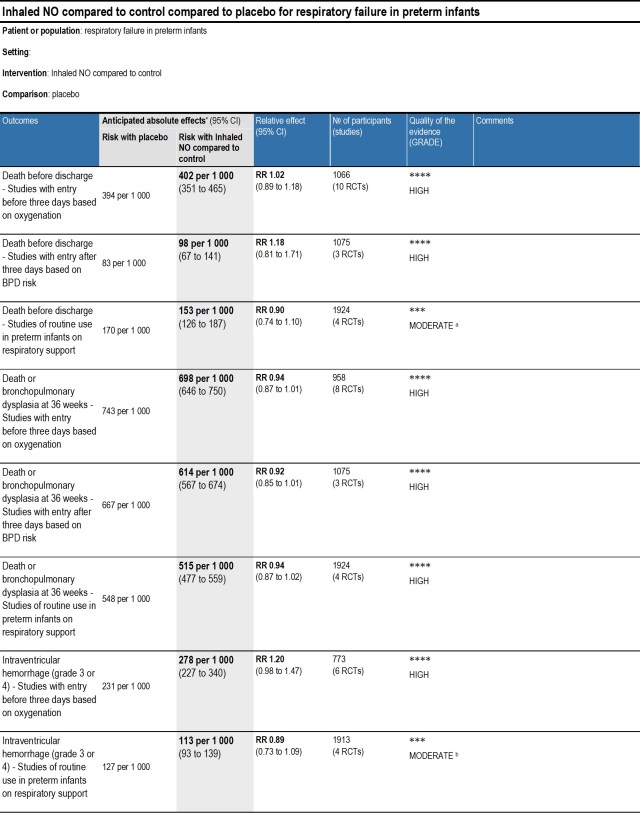
The Summary of Findings tables are newer, and follow GRADE standards for determining how much confidence we have in the findings. This is the SoF table for the new version of this review.
As you can see, the summary is that there is no evidence of benefit from the use of inhaled NO for any of these indications in the preterm. The situation where the potential benefit is closest to being statistically significant is the use of inhaled NO for infants older than 3 days who are determined to be at elevated risk of BPD. One of those studies was somewhat different to the other 2 in that category, the study by Subhedar, but that trial only contributed about 40 of the over 1000 subjects, so we did a sensitivity analysis, eliminating that study, which still shows an effect consistent with chance.
I also applied the Barrington rule, which is that if it was not possible to be eligible for 2 studies, they shouldn’t be meta-analyzed, Subhedar’s study included babies at 96 hours, the other two studies were minimum 5 days of age, and those two had considerable overlap in eligibility. So the 2 larger studies satisfy the Barrington rule, but not the smaller one.
So is this the final word? Does this mean that it is never justified to give inhaled NO to a preterm infant? My response to my own question is that I think that the evidence is consistent with a statement such as “the data currently show that the administration of inhaled NO to critically ill preterm infants in the first few days of life, with hypoxic respiratory failure, does not lead to an overall increase in survival”. That is not the same as saying that there are never any indications for a trial of inhaled NO, especially when there are clear indications of pulmonary hypertension.
Some groups of preterm babies have not been adequately studied, for example, preterm infants with clear evidence of pulmonary hypertension. Such infants may have a poor response to surfactant, and have a severe pre- to post- ductal oxygenation gradient, they often have a dramatic immediate response to inhaled NO, just like babies at term. It seems unreasonable to withhold NO from such a baby at 33 weeks gestation, when an otherwise identical baby at 35 weeks gestation would receive the treatment. Our individual patient data meta-analysis suggested that there may possibly be a benefit to using inhaled NO in preterm babies with pulmonary hypertension, but the interaction term was not statistically significant, and there were very few babies for whom pulmonary hypertension had been recorded in their original data files, and there was not a clear, consistent definition of pulmonary hypertension either.
I do think there is room for more trials in the preterm. An ethically justifiable trial would include preterm babies with good evidence of pulmonary hypertension, and would allow rescue treatment in the controls if they continued to be hypoxic after an interval, such as 60 minutes. Current data show a high frequency of intracranial hemorrhage in preterm babies with apparent pulmonary hypertension who receive NO; and I think it is possible that the sudden improvement in pulmonary vascular resistance, and sudden increase in cerebral perfusion that accompanies this change, could cause an increase in IVH. It might be that just being more patient, and awaiting a more gradual diminution in pulmonary artery pressures may lead to better outcomes. Hence a trial would be appropriate, and I think needs to be done. My opinion is not the same as the group that published some recommendations in March last year (Kinsella JP, et al. Recommendations for the Use of Inhaled Nitric Oxide Therapy in Premature Newborns with Severe Pulmonary Hypertension. The Journal of pediatrics. 2016;170:312-4) they stated that performing an RCT in such babies was not feasible, but I don’t understand why not. We have done studies in near-term babies who were near-death, and proved the value of inhaled NO. All we currently know in the preterm baby with PPHN and inhaled NO is that many of them have acute short-term responses, we have no evidence of improvement in survival or serious long-term consequences.
The other group of preterm babies for whom inhaled NO is currently frequently used are those babies who are developing severe chronic lung disease and go through periods of very poor oxygenation. Those babies also frequently have at least a partial response to inhaled NO, and I think a trial in such babies to determine if NO is actually beneficial for clinically important outcomes would also be justified.