As usual the PAS meeting was packed with neonatology. The App was even worse than last year, crashing frequently on many peoples’ phones. It was completely useless for the posters, as they were listed by number, and the different sessions all started with #100; so there might have been 3 posters with the same number and no indication of which day they were on.
The organisers also decided to not have any indication of what subjects were in various areas of the poster session, so you might accidentally happen upon a group of posters about breast milk and breast feeding, for example, but there wasn’t any way to know that such a group existed, or how to find them. As there were several hundred posters in each session, it was impossible to actually find the things that interest you. If I wanted to see the posters about neonatal respiratory care, I just had to wander around the huge room and try to find them, the App didn’t help at all.
Sunday morning was very interesting, with Neonatal Clinical Trials 1 followed by the Silverman lecture given by Dr Annie Janvier then Neonatal Clinical Trials 2. Unfortunately there were other interesting neonatal things going on at the same time, which had tiny participation, a session on BPD and another on caffeine would have been super interesting, but I would guess had almost no-one there, as the Clinical Trials sessions were packed.
As for those Clinical Trials, I will outline a few of them here and give a link to the protocol if one is published, and may well return to them with a longer post when they are actually published. One of them was the Video-Laryngoscopy trial that I have already posted about. In no particular order the others were :
The PLUSS trial
The PLUSS trial showed that budesonide is not a plus when added to curosurf. Many of you will know that previous work, including moderately large trials from Taiwan, have suggested a reduction in early signs of lung injury when initial surfactant dosing was mixed with budesonide, comapred to budesonide alone. This trial randomized infants <28 weeks receiving surfactant either by intubation or LISA/MIST, and followed the babies until discharge.
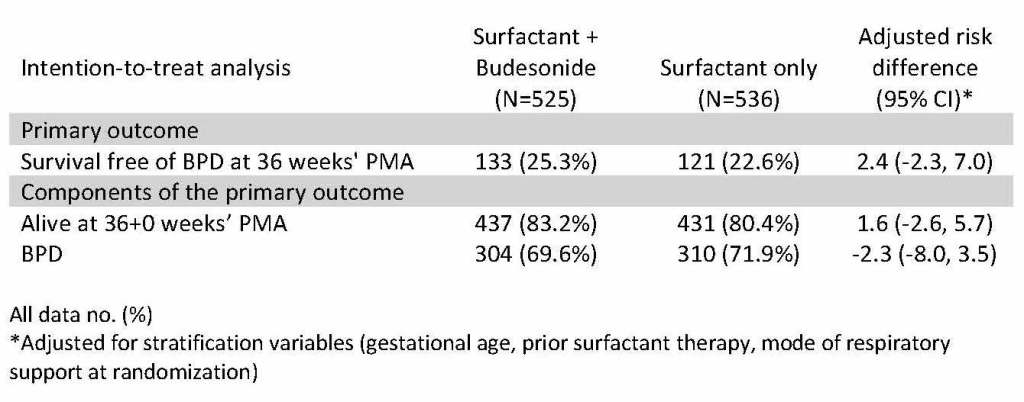
As you can see there was no impact on either BPD at 36 weeks, or survival to 36 weeks. I think it is very unlikely that any longer term follow up will show a benefit from the budesonide, although there may be a risk of neurodevelopmental adverse impacts form this potent steroid, a small proportion of which is absorbed. There was no subgroup that appeared to have any benefit. A recent observational study has suggested that the budesonide only seemed to help the larger babies, perhaps that is the difference to the prior positive trials, which included many larger infants.
IBUPAR
The IBUPAR trial was a multicentre Spanish trial including preterm infants <30 weeks with a “haemodynamically significant” PDA, to either Ibuprofen or Paracetamol (=acetaminophen), which was diagnosed on average about 3 to 4 days of age. I found it odd that this was being presented, as the sample size is 274, but only 134 have completed the trial and 111 completed the treatment, nevertheless the data from an interim analysis have been unmasked in order to be presented at PAS. The primary outcome is closure of the PDA, which occurred in 60% with ibuprofen, and 40% with paracetamol. By the time of discharge most had closed in both groups, but it was 85% in the ibuprofen group and 70% with paracetamol. Acute Kidney Injury was much more frequent with ibuprofen, 16% vs 5%, but I am not sure what the diagnostic criteria were for that diagnosis, whether it was a transient oliguria, or persistent increase in creatinine, or some combination. There were no major differences in NEC, BPD, IVH, ROP or mortality. This shows I think that it doesn’t matter much if you give a less effective therapy to close the PDA, the babies turn out just as well despite having an open PDA.
There were two trials of prolonged use of caffeine in the preterm.
The MOCHA trial
The first was the MOCHA trial, which unfortunately. despite the name, did not give the babies chocolate along with their caffeine. In this trial 827 moderately preterm babies (29 to <34 weeks GA) who had received caffeine for clinical reasons, and had arrived at 33 to <36 weeks PMA, with a plan to stop their caffeine, were randomized to continue caffeine citrate at a dose of 10 mg/kg/day or placebo, with the plan to continue until and beyond discharge, for a further 28 days at home. The primary outcome was the number of days of hospitalisation, the idea being, of course, that less apnea, and fewer hypoxic events will lead to earlier discharge. There was no real difference in the primary outcome, which was slightly shorter in the placebo group, and identical after adjustment. Days to “physiologic maturity” (which meant apnea free, full oral feeding and stable in a crib) was also identical. Caffeine was having an effect, however, the caffeine group babies had fewer apneas, it was 2 days faster for them to be 5 days apnea free, but in the end it wasn’t apnea that kept most babies in hospital but feeding issues. There were no adverse effects, so in terms of duration of hospitalisation, caffeine is likely to only have an impact in the subgroup who are having persistent apnea.
The ICAF trial
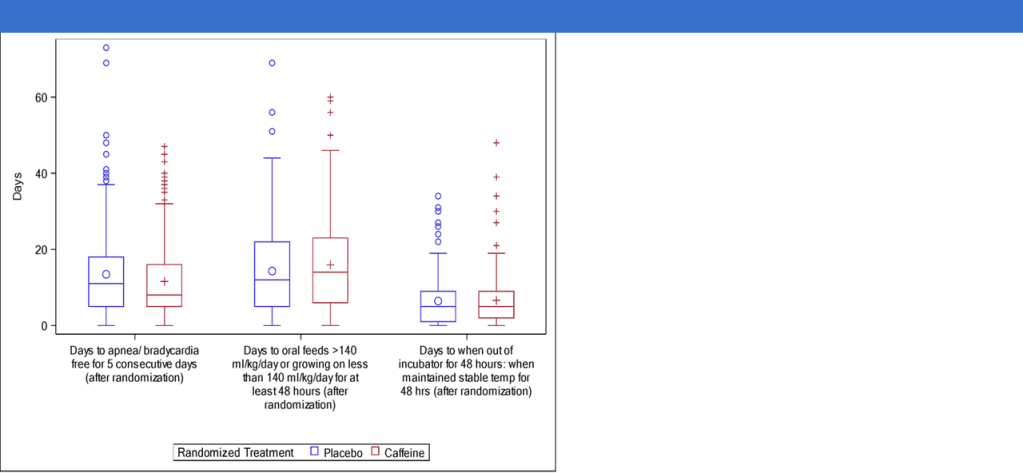
On the other hand, the ICAF trial enrolled more immature babies <30 weeks GA at birth, who had arrived at 32 to <37 weeks and were about to have their caffeine stopped. Within 3 days of caffeine being stopped they were randomized to caffeine or placebo at the standard dose, but when they reached 36 weeks the dose was doubled to 20 mg/kg/day of caffeine citrate. The babies were on recording pulse oximeters until 44 weeks, and stayed on caffeine or placebo until 43 weeks. 160 babies were in the study, and these are the post-randomization findings.
| Characteristic | Placebo N = 78 | Caffeine N = 82 | P-value |
| Clinical caffeine | 3 (3.8) | 3 (3.7) | 0.95 |
| Supplemental oxygen | 14 (17.9) | 4 (4.9) | 0.009 |
| CPAP, BIPAP or High Flow NC | 7 (9.0) | 2 (2.4) | 0.07 |
| Mean (SD) PMA (weeks) at discharge* | 38.5 (2.2) | 37.7 (2.2) | 0.04 |
| Mean (SD) weight gain (g/day)* | 38.5 (25.3) | 29.2 (12.7) | 0.004 |
| Median (min – max) days from randomization to discharge)** | 27 (21 – 32) | 17 (15 – 23) | 0.001 |
In these more immature babies caffeine did shorten hospitalisation, and improved their respiratory course, not just in terms of apnea, but by keeping them off oxygen or respiratory support. The oximeters showed dramatic effects on intermittent hypoxia, which continued to be significantly different until 42 weeks.
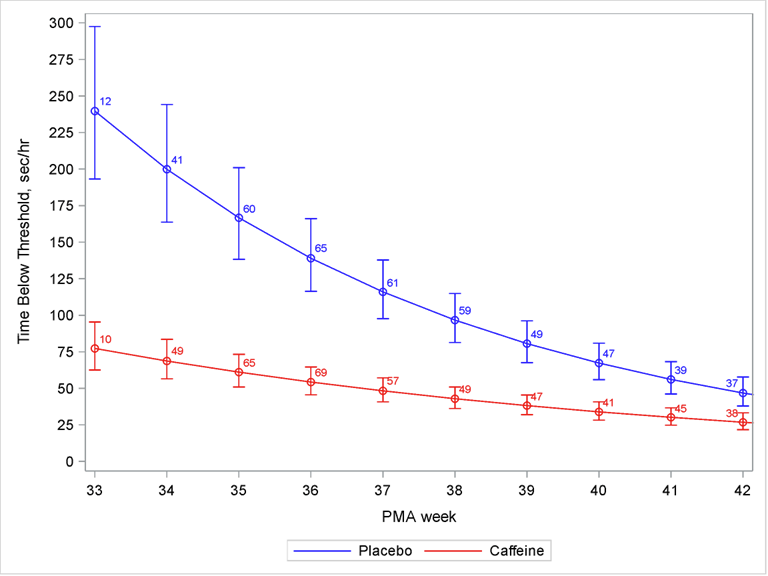
I think the big difference between the two trials is the less mature group enrolled in ICAF, who have more IH for longer, and have more lung disease.
We already know that caffeine metabolism changes at near to term, and levels are very low on the standard dose, so I think the increased dose was a good idea in ICAF. But I don,t think it would have made much difference to MOCHA as it wasn’t apnea, or IH that were keeping the babies in hospital.
Long term follow up will be important for both trials, and ICAF2 with a larger sample size is certainly warranted based on this information.
Long term follow up of BABY-OSCAR
I posted about the short term results previously, it was an RCT of early closure of the PDA with ibuprofen that had basically a null result. The infants were followed at 2 years of age, with parental report of their development using a scale called PARCA-R, unfortunately the outcome was dichotomised into those with moderate or severe “neurodevelopmental impairment” or without. There were slightly more overall deaths with ibuprofen, and slightly more so called “impairments”, most of which I presume was slower development, in the controls who survived.
Longer term respiratory outcomes were also presented, including duration of oxygen treatment from randomization to stopping oxygen, and a composite of respiratory morbidity, the definition of which is shown in the legend to the figure below. It shows not a hint of any difference.

Trial of Prolonged CPAP therapy
Finally, in the first session in the morning there was a trial of prolonged CPAP therapy, which disconcertingly had no zippy acronym. This should be illegal, how can a poor blogger think up catchy titles for their posts? Babies of 24 to 32 weeks GA at birth who were on CPAP, who were thought to be ready to stop their CPAP and who met stability criteria, had FRC measurements and were then randomized to have 2 extra weeks of CPAP at 5 cmH2O, using a bubble system.

The lungs grew more on CPAP. As you can see, after the extra 2 weeks the FRC was close to 20% greater in the prolonged CPAP group. At 6 months of corrected age lung volume was again measured, with a different technique which gives alveolar volume, and they also measured the diffusion capacity for CO,

At 6 months the prolonged CPAP treated lungs has a higher alveolar volume, and had better gas exchange.
I think this is potentially a major advance in respiratory care, it may be that babies who are at high risk of long term respiratory problems might be helped just by leaving them on CPAP for two weeks. Of course we don’t know if they had better or worse small airways function, or any difference in any clinical outcomes. But, many of us have major concerns about the very long term outcome of our patients, who may have deteriorating lung function later in life if they start out with fewer alveoli. This is potentially a big advance, if we can show that it is safe and has medium term advantages.









Hi Keith — for the last trial, it was called the eCPAP Trial by the investigators — it is kinda zippy 🙂