I like a good acronym, so my initial response to the new TORPIDO trial was very positive! TORPIDO 30/60 was a large multicentre RCT comparing initial FiO2 concentrations for resuscitation of the preterm infant (Oei JL, et al. Targeted Oxygen for Initial Resuscitation of Preterm Infants: The TORPIDO 30/60 Randomized Clinical Trial. JAMA. United States2025). The first TORPIDO study (Targeted Oxygen in the Resuscitation of Preterm Infants and their Developmental Outcomes) was a comparison in 290 infants <32 weeks GA, of 21% starting O2 concentration vs 100% starting concentration (Oei JL, et al. Targeted Oxygen in the Resuscitation of Preterm Infants, a Randomized Clinical Trial. Pediatrics. 2017;139(1)) . It had to be stopped early because it became difficult to enroll patients as we became reluctant to use 100% oxygen. Although underpowered, there was a numerically greater mortality in the 21% oxygen group, especially among the infants <28 weeks.
This contributed to an apparent increase in mortality among lower FiO2 regimens when all the Individual Patient Data were included in a Network Meta-Analysis, NETMOTION. (Sotiropoulos JX, et al. Initial Oxygen Concentration for the Resuscitation of Infants Born at Less Than 32 Weeks’ Gestation: A Systematic Review and Individual Participant Data Network Meta-Analysis. JAMA Pediatr. 2024).
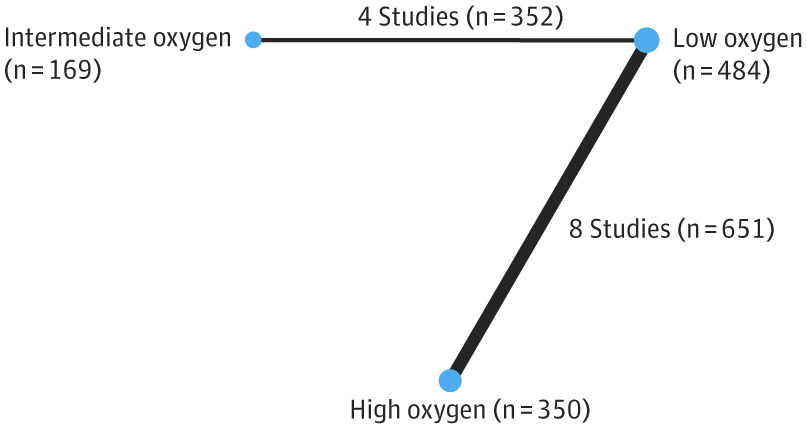
That NMA suggested that the higher starting oxygen groups (90% or higher), had a lower mortality than low starting concentration (30% or less), while intermediate, >30% to <90%, was similar to low starting concentration. Of note, the 1st TORPIDO study was the largest in that NMA.
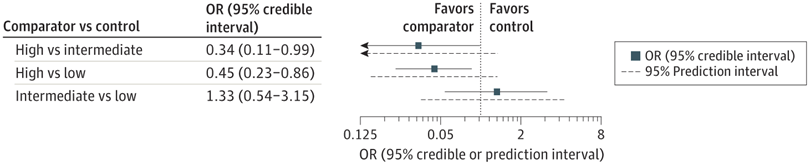
Unfortunately, 10 of the 12 trials in that NMA were very small, 31 to 95 subjects, the 2 larger ones were modestly sized (287 and 193). For such an important outcome, and an easily applied, no-cost intervention, better, larger trials were needed.
The new TORPIDO 30/60 trial started in 2018, well before NETMOTION was performed. According to the choices made by the authors of NETMOTION, it would have been considered a comparison of intermediate (60%) to low (30%) oxygen. At the time of designing it, it was considered unlikely that very high starting oxygen concentrations would be a good idea, so the choice of a comparison between 30 and 60% was entirely reasonable.
The new trial results show no difference in the primary outcome, or in any indices of brain injury on ultrasound.
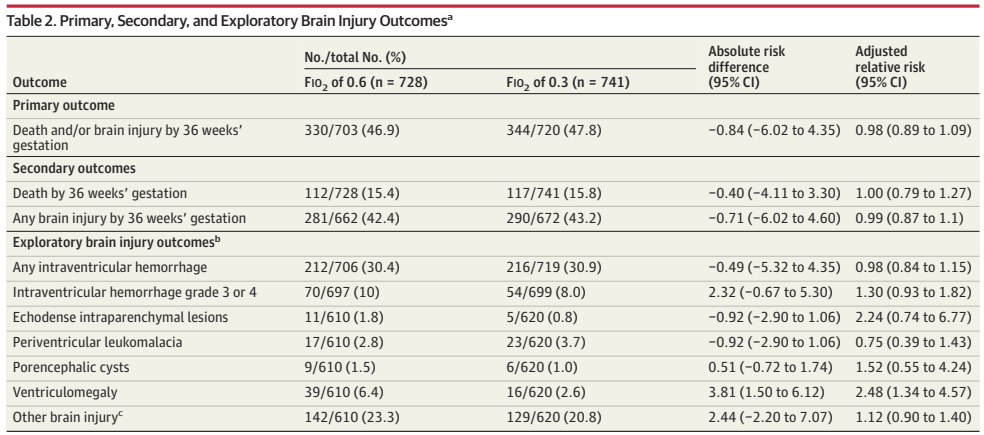
There were some differences in immediate responses in the delivery room, “Newborns in the FIO2 of 0.6 group, compared with the FIO2 of 0.3 group, were less likely to receive chest compressions (2% vs 5%) or epinephrine (1% vs 2%) and more likely to reach SpO2 > 80% by 5 minutes (58% v 44%) (Table 3) and had higher initial SpO2.” Which are interesting differences, but did not translate to any measurable difference in clinical outcomes.
This suggests to me that we have to push this further, and compare a starting FiO2 of 60% (which seems no worse than anything lower, and might have some short term advantages) to a starting FiO2 of 90 or 100%. It seems weird, after all this time, to talk of going back to 100% oxygen for initiating resuscitation. It seems only a few years ago (but I guess it is more than 2 decades now) that I had to approach the hospital administration at my previous post to have pressurized room air and air/oxygen mixers installed in the delivery room, so that we could start resuscitation with 21%, and titrate if needed. The DR, in a relatively new building, had been built with just piped oxygen for neonatal resuscitation. The administrators of the Royal Victoria Hospital in Montreal were very responsive, and immediately approved the budget when I presented the evidence.
Most of my readers will know that the evidence for room air resuscitation in full term infants was quite strong, and that routinely starting with 21% oxygen was associated with a 27% lower mortality than 100 % oxygen, as this ILCOR meta-analysis showed. https://publications.aap.org/pediatrics/article/143/1/e20181825/76868/Room-Air-for-Initiating-Term-Newborn-Resuscitation
But if the data for preterm infants is taking us back to much higher oxygen concentrations, I guess we will have to decide whether 34 week infants should be treated like the 35 weekers, or like the more immature infants. Just like my comments about cooling, I think it is unlikely that the risks change dramatically at midnight between 34 weeks 6 days, and 35 weeks. We will need more granular information in order to make decisions for infants in the late preterm age ranges. Hopefully. those doing the trials will provide Individual Patient Data, that will allow analyses according to gestational age, and other risk factors.









The question then is .. at what specific criteria does one rack up the oxygen.. apart from a vague….not responding sufficiently… or is that sufficient ?
If we return to a starting FiO2 of 90 or 100% then the question will become at what specific criteria does one decrease the oxygen! Currently we try and aim to have the SpO2 within the Dawson percentiles, but exactly how we should adjust the oxygen… Should we rack up the concentration to 100% immediately if the infant is more than 10% below the lower limit? Or increase sooner, but more progressively? I don’t know the answers to any of this, and they would be extremely difficult questions to operationalise. Maybe we should be training AI models, with recordings of FiO2 and SpO2 etc, perhaps then an AI could control the FiO2 to give the best way to most quickly achieve the sats that we want. Failing something like that, I guess the guidance will remain to start at a particular FiO2, when we have figured out what it should be, and adjust the FiO2 according to response.