In some pre-clinical models, erythropoietin acts as a neuroprotective agent, which led to the performance of clinical trials to determine whether there was a positive impact on outcomes of the preterm. The latest study has just been published, and it is another high quality trial from the NICHD NRN, and Robin Ohls (Ohls RK, et al. Darbepoetin, Red Cell Mass, and Neuroprotection in Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr. 2025). The article starts with a questionable assertion, “Erythropoiesis-stimulating agents (ESAs) such as erythropoietin…have shown evidence for neuroprotection in preterm infants”. I think if that was true, they would not have done this trial! In fact the references they give for that statement show a very variable impact on outcomes, the figure below is from a SR/MA which included only erythropoietin studies, eliminating a darbepoietin (a long acting EPO analogue) group in the second study by Robin Ohls et al (2014).
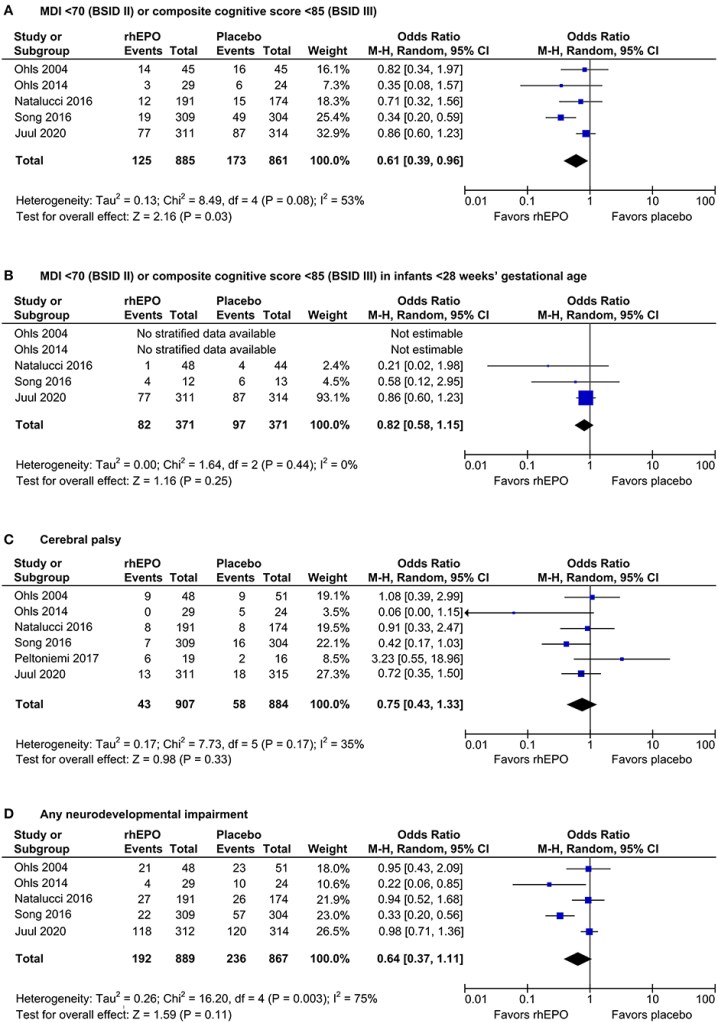
This is not a criticism of this trial, or of the justification for performing it, which I think was very well founded, just the way that sentence is worded.
It is very important to note that the possible benefit of EPO on long term outcomes is entirely dependent on Song 2016, which was retrospectively registered after completion, and published in contravention of the guidelines of the journal where it was published. If you delete the Song data there is very little indication of a benefit of EPO on the long term.
The highest quality trial among those analyzed in the SR/MA was Juul, which showed nothing, in terms of impacts on long term neurological or developmental outcomes. The Natalucci, Swiss EPO trial, was also high quality, and about 2/3 the size, and was also without long term benefit.
The new trial enrolled 650 infants between 23 and <29 weeks GA, <36 hours of age, and continuing until 35 weeks PMA. Darbepoietin 10 mcg/kg or placebo was given weekly, IV while the access was in place followed by subcutaneous Darbe, or a sham procedure as placebo.
The primary outcome, I am very happy to say, was NOT “death or NDI”!!!! The primary outcome was a continuous variable, the Bayley version 3 cognitive composite, with dead infants being assigned as score of 54, which is the lowest possible score. I’m not sure this is the best approach, as the difference between a score and 55 and 56 is the the same as between 55 and being dead. As there was no imbalance in the deaths (50 in each group during hospitalisation, and 2 Darbe vs 3 controls after discharge) it probably changes little how exactly the deaths were accounted for, in terms of the difference between groups.
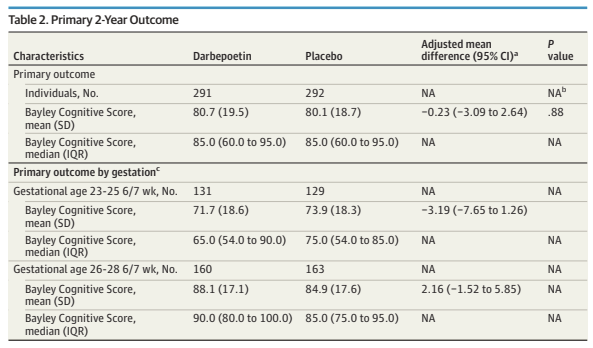
On the other hand, the scores seem lower than they are if you calculate them solely among the survivors, which results are given in the supplementary appendix.
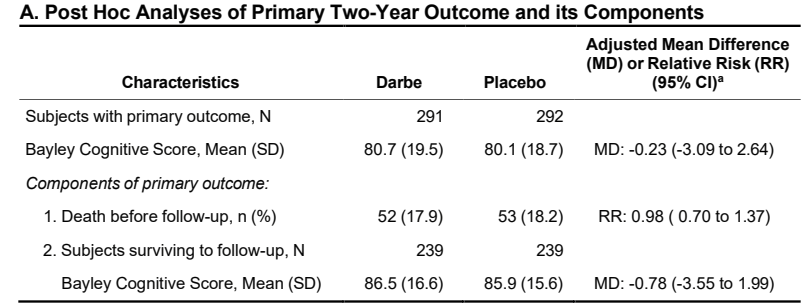
And the higher mortality among the more immature group has a greater impact on the apparent cognitive scores in that group
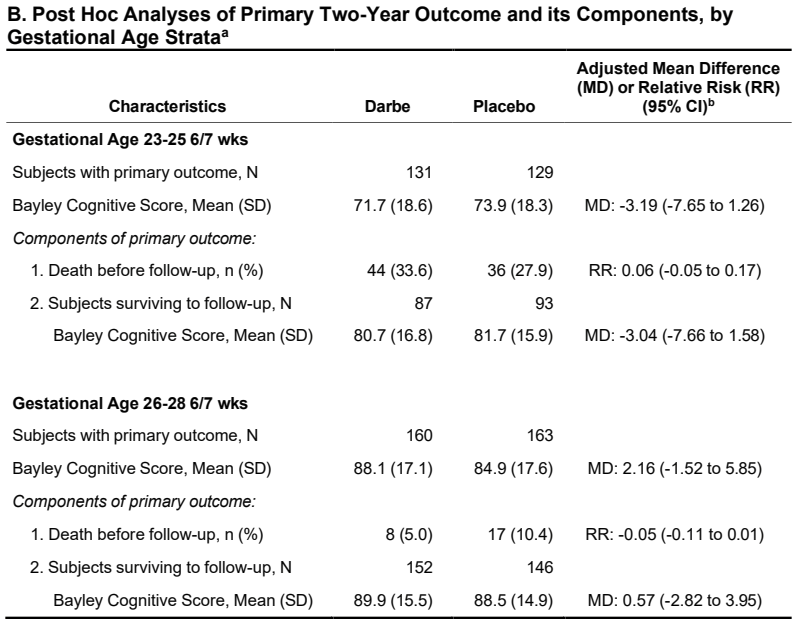
Even though hidden away in the supplementary appendix, that is the result that I think is most interesting; among survivors what was the impact of darbepoietin on cognitive development at 2 years corrected age?
The supplementary appendix also shows that the other components of the Bayely scores were similar between groups, and that there was no difference in neurological impairment (cerebral palsy or visual or hearing impairment).
As for other impacts of darbepoietin prophylaxis, the intervention was very effective. It stimulated bone marrow red cell production, increasing haemoglobin, reducing transfusions (with a transfusion guideline which was the same, of course, for the 2 groups), and reducing donor exposure.
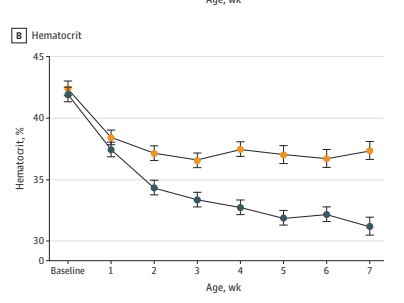
This is part of the figure showing that darbepoietin, yellow circles, increased haematocrit compared to the blue circle controls, despite fewer transfusions.
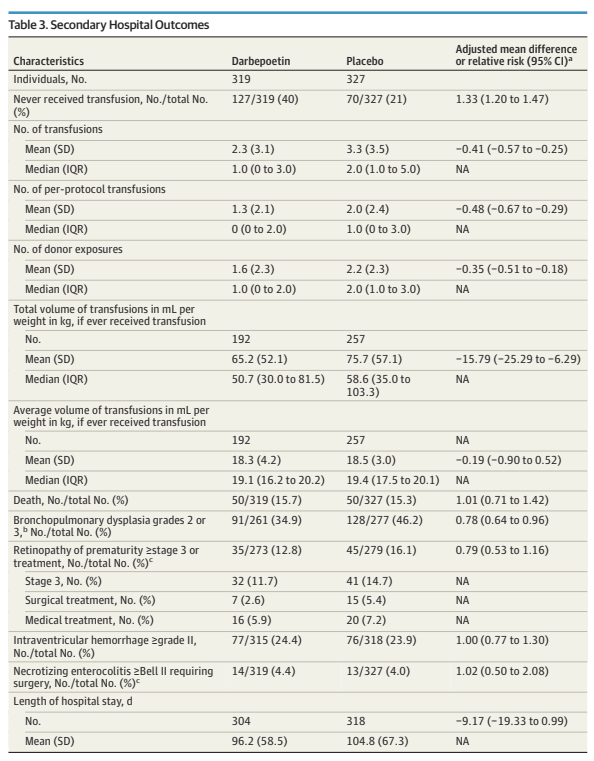
Darbepoietin doubled the proportion of babies who never had a transfusion, decreased the total number and volume of blood transfused, and decreased the number of donor exposures.
It is also interesting that this did not affect the frequency of surgical NEC, and there was less severe BPD, and probably less severe retinopathy.
The lack of impact on surgical NEC is interesting and consistent with all the other studies, and is another evidence point against the existence of so-called TRaGI, Transfusion-associated Gut Injury. To explain, there is an idea that transfusions trigger NEC in preterm infants, and that how we feed infants around blood transfusions might have an impact on the complication. But if you look at all the data, there is no evidence from prospective studies of transfusion thresholds, or of EPO and similar agents, that fewer transfusions leads to less NEC. I think that is the best evidence we have that the association between transfusion and NEC that has sometimes been reported is just coincidental.
The reduction in severe BPD was not seen in the PENUT trial, nor in the large Swiss EPO trial, so might well just be a random finding, but, at the very least, there is no evidence of increased lung injury with these agents. Similarly with retinopathy, there is generally a tendency to have somewhat fewer cases.
I’ve never seen an analysis of the cost benefit of these agents, but the dose we give to babies is tiny (a quick search looks like it costs about 3 dollars per microgram in Canada), and the costs of transfusion are substantial, even though blood in most countries is donated freely by heroes, the total cost of a transfusion in Canada was recently estimated to be about $500. I think it is likely that darbepoietin is cost-effective, and, if I was a parent in the NICU currently, and given the choice of darbepoietin compared to an increased risk of transfusion, I think I would choose darbepoietin. The only downside is the pain of a weekly subcutaneous injection, which is the main advantage of darbepoietin, compared to erythropoietin, which requires painful administration 3 times a week.
I think that based on all these recent data, that erythropoietin and analogues are safe, in the short and the long term, effectively increase red blood cell production, and reduce blood transfusion in a clinically significant way. Their use should be standard of care for very preterm infants, or offering their use to parents should be standard practice. Just don’t expect them to improve neurological or developmental outcomes








