I used to do a series of shorter posts called “weekly updates” but I ran out of steam and have concentrated on longer posts in recent years. The last couple of weeks, with clinical service, I have had less time for a longer post, but a few things piqued my attention:
Alexe PI, et al. Prematurity and Congenial Diaphragmatic Hernia: Revisiting Outcomes in a Contemporary Cohort. J Pediatr. 2025:114545. I thought I would write about this article before the title got corrected, (unless, that it, they really are discussing “congenial” diaphragmatic hernia). Right now if you do a search on “congenial” in pubmed, you get this hit and a couple of auto-mis-corrected titles!
There was also an article about congenial cataracts, and several others about congenial heart disease. The pre-print of the article has the error right on its front page, it has probably been seen by hundreds of pairs of eyes, and everyone’s brain auto-re-corrected it back to congenital! I am not writing about this just to poke fun, however, it really is a very interesting piece of work from an international consortium, reporting analysis of over 13000 cases. It demonstrates very clearly the major impact of prematurity on management and outcomes of CDH. Survival of all cases progressively increases up to 40 weeks gestation.
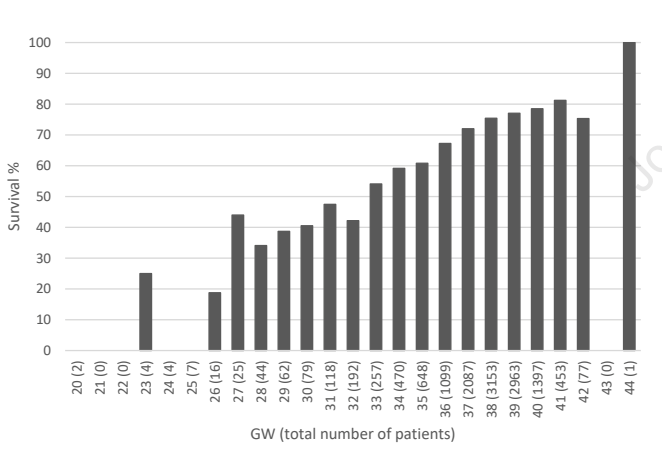
For infants of <34 weeks gestation, large numbers never have a surgical repair, nearly half, and those that do, get their surgery much later, at 11 days mean, compared to about 1 week for the early term and term groups. ECMO was used in about a third of the cases after 34 weeks, but only 7% of the <34 week infants. ECMO survival was 30% for the <34 weeks, 40% for the late preterm, and just over 50% for the term infants. All of the measures were more marked for the very preterm, and worse again for the extremely preterm.
Within the database there are many infants who delivered after fetal intervention (FETO), which was associated with lower survival. This was clearly because they were a much higher risk group, which is why they were selected for FETO, overall survival was only 54% after FETO, compared to 71% among the remaining infants, and almost all of the FETO babies needed a patch repair. FETO was also associated with increased prematurity, and among those who did deliver preterm, survival was poor, about 40%, but was identical with or without a prior FETO.
Take Home Message : Prematurity is very bad news for infants with CDH.
Franz AR, et al. Effects of liberal versus restrictive transfusion strategies on intermittent hypoxaemia in extremely low birthweight infants: secondary analyses of the ETTNO randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2025. This is a secondary analysis of the ETTNO trial, the primary publication of which which showed no overall benefit of a higher versus a lower transfusion threshold in the very preterm infant. This analysis focused on any potential impact of transfusion threshold on apnoea spells, which are the usual pathophysiology behind intermittent hypoxia. Multichannel recording and analysis of apnoea patterns, which I have done, and published, many hundreds of times and published is much more difficult and time consuming than just recording oximeter saturation data. In addition, unless you record airflow as well as just respiratory movement and heart rate, you gain very little, and measuring airflow is tricky. Most recent studies therefore have measured, and discuss, intermittent hypoxia.
I actually started an RCT of transfusion therapy for apnoea when I was in San Diego, we enrolled only about 20 patients prior to me leaving UCSD, and the fellow also graduated, so the trial ground to a halt unfortunately. I did have a poster at an SPR about our preliminary findings, but never felt that it was worth a publication. Basically we performed multi-channel recordings, including an expired CO2 detector next to the nose of the infants, among convalescent preterm babies with apnoea and a hemoglobin <100. We randomized them to get an immediate transfusion if the attending physician thought it was reasonable to transfuse, or to not be transfused for at least 72 hours, and did physiological recordings for 72 hours. The trial was of course therefore very underpowered, but there was no impact of transfusion on the respiratory patterns of the babies.
We did that trial because, at the time, recurrent apnoeic spells were often as an indication for transfusion in anaemic preterm infants. There were also a few observational cohorts, which suggested that apnoea becane less frequent after transfusion, but those cohorts were all uncontrolled. Control groups are essential for many things, but especially with a condition such as apnoea of prematurity, which always gets better with time anyway! Without a control group, any intervention that you can imagine will be followed by decreased apnoea, especially when you factor in that babies are usually enrolled in a trial when their apnoea frequency is at its highest. I was rather sceptical about apnoea as a transfusion indication, which was the justification for the RCT.
This new publication has compared intermittent hypoxia episodes, occurring between days 8 and 49, between infants in the higher transfusion threshold group to the lower group. All the babies were less than 1kg, and there were over 250 per group.
There was no difference between the groups in numbers of IH episodes, duration of episodes, more severe episodes, or the median duration of episodes. Or indeed, as they have previously published, on survival or neurological or developmental outcomes. There was also no interaction between IH, transfusion strategy, and outcomes.
Take Home Message : Intermittent hypoxia, and/or apnoea of prematurity is not an indication for blood transfusion.
Cheung PY, et al. Dose-related systemic and cerebral hemodynamic effects of norepinephrine in newborn piglets with hypoxia-reoxygenation. Pediatr Res. 2025. Po-Yin Cheung and the group in Edmonton refer to the lab where they do their animal work, as my old lab; I set up many of the processes and bought some of the equipment that they are still using 30 years later, including the Transonic flow probes! Po-Yin, and Georg Schmolzer, and their colleagues, have gone far beyond what little I accomplished there, however. This new publication is in acutely instrumented anesthetised newborn piglets, and investigates the haemodynamic and cerebral circulatory effects of norepinephrine infusions. The model they use is one developed by Po-Yin, of hypoxia and re-oxygenation, and is designed to be a model of infants after perinatal asphyxia.
Our group in Montreal have been using norepinephrine for circulatory support for a few years now, mostly for septic shock or for infants with PPHN who need support. In both circumstances there is a small amount of animal data, and a very small amount of human neonatal data suggesting advantages over other agents. One thing that has been missing is a good idea of what the agent does to the cerebral circulation, being very difficult to accurately measure cerebral perfusion in the newborn infant.
The model used in this study leads to a severe metabolic acidosis, lactate of 20 and Base Deficit of 20, and 2 hours later, prior to the study medications begin administered, the acidosis was a little improved. Animals then got either saline, or epinephrine infusion at 0.1 mcg/kg/min or one of 3 doses of NorEpinephrine (0.05, 0.1 or 0.2 mcg/kg/min). Without going to all the details of the findings, NE at 0.1 mcg/kg/min had the biggest positive impact on cardiac function and cardiac output, With cardiac output increasing by 100%, due to a small increase in heart rate, of about 20%, and a major increase in stroke volume. At the same time systemic blood pressure increased by about 20%.
In addition carotid blood flow increased by nearly 20% leading to an increase in cerebral oxygen delivery and brain oxygenation.
Clearly, one cannot directly extrapolate these results to the human newborn, and particularly not specific dose responses, but they do give some reassurance that norepinephrine does not vasoconstrict the neonatal cerebral circulation.
Take Home Message: (with lots of caveats) norepinephrine infusions may lead to improved cerebral oxygenation.
Fox L, et al. A Pilot Randomized Control Trial of Holding During Hypothermia and Effects on Maternal and Infant Salivary Cortisol Levels. Adv Neonatal Care. 2025;25(2):173-80. Many centres, including ours, have instituted “cool cuddles” (câlins-frisquets in Quebecois), whereby infants with HIE being cooled are allowed to be cuddled by their mothers (or fathers!). We have demonstrated, for our own purposes, that this doesn’t adversely impact temperature control. We still have a few restrictions, for infants with multiple central lines it gets quite difficult to ensure safety. Although it seems like the right thing to do, from first principles, this new randomized trial sought signs of infant stability and stress, and maternal stress from their salivary cortisol levels. Mothers in the cuddling group had lowered cortisol, as did their infants, who also showed lower, stable heart rate and respiratory rate, without desaturation, or temperature problems.
Take Home Message : Cuddling during therapeutic hypothermia reduces maternal stress.








