Since at least 2013 the World Medical Association Declaration of Helsinki has mandated registration of clinical trials prior to enrolment of the first subject. Since 2005 the International Committee of Medical Journal Editors has required trial registration as a condition of publication, before the onset of patient enrolment.
This principle is reiterated in the new version of the declaration of Helsinki, just published and discussed in many places, including JAMA.
Nonetheless, retrospectively registered trials are still being accepted by journals, most of whom should know better, as well as publications with no mention of registration at all. It has been a recurrent problem in some neonatal studies, and some large recent neonatal trials were retrospectively registered after being completed, and after all the data were collected. The fact that prominent journals will still sometimes accept such research gives the publications a credibility which is not deserved.
This has a significant impact on how we analyse the literature and make therapeutic decisions. To take one example, the use of Erythropoietin in preterm infants. Let’s analyse the literature to determine if Epo prophylaxis decreases NEC, a major health issue in these babies. A recent high-quality systematic review was published by Ananthan et al, Ananthan A, et al. Early erythropoietin for preventing necrotizing enterocolitis in preterm neonates – an updated meta-analysis. Eur J Pediatr. 2022;181(5):1821-33. This SR found 22 studies with over 5000 patients enrolled, and a clinically significant reduction in “definite” NEC, RR 0.77 0.61-0.98. However, of the 4 largest trials in that review 2 were registered after completion of enrolment (Song 2016, n=743, Wang 2020, n=1285), one was registered in 2006, shortly after the first patients were enrolled in 2005 but prior to completion (Natalucci 2016, n=365) and one was registered prospectively (Juul 2020, n=936). If we re-analyze the data excluding the retrospectively registered data, and the older non-registered trials, the effect of Epo on NEC no longer reaches classical statistical significance
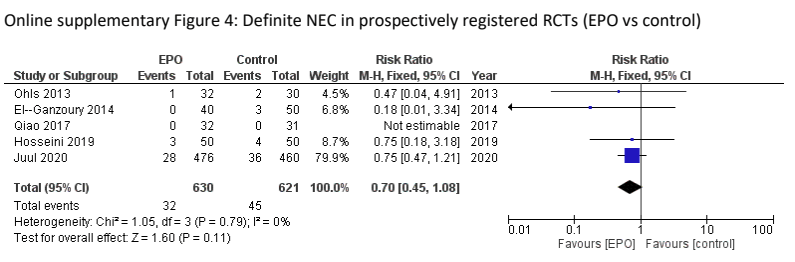
It does still look like there might be an effect, doesn’t it? Adding Natalucci back in (the absolute requirement for prior registration dates to around the time the study was started, so it is much less of an issue) makes little difference, as they had only 3 cases with Epo and 4 in the controls. Which points out how desperately important this is. Are we failing to provide an effective therapy for our babies? Are we at risk of dosing thousands of babies with a prophylactic intervention which is ineffective?
One characteristic of many of these retrospectively registered trials is they are not published in the typical neonatal/pediatric literature, Wang 2020, for example was published in the Journal of Translation Research. Song 2016 was published in Annals of Neurology, which claims to follow the ICMJE guidelines and the declaration of Helsinki, but clearly failed to do so for this article.
I’m not sure what to do about this on-going problem. If it was bench research we could just refuse to publish, but when babies have been subjected to research procedures and parents to the stress of having a baby in an NICU and in a trial, it seems unfair to those research subjects to categorically refuse to publish. But, unless there is some sort of sanction, this will continue, and indeed it does continue. Perhaps there should be a separate journal: “The Journal of Unregistered Research”, or a separate section in some journals: “Unreliable Information”. We can’t ascribe ignorance to the authors, Song et al, who retrospectively registered the first trial in 2016 are the same authors as Wang et al, who retrospectively registered that later trial in 2020! There are numerous other problems with these 2 projects, they were unmasked trials but the controls still received placebo injections, why on earth would you do that?
The importance of trial registration is discussed in many different places, including by the WHO, one thing that the WHO don’t discuss, but which is vitally important, is that prior trial registration makes it clear if primary outcomes have been changed, or if outcome definitions have been “adjusted”. Even better is full publication of the trial protocol, in addition to registration, which makes those things even clearer, and less liable to fudging the results.
The problem is not, it seems, going away. Which may, in part, be because of the proliferation of biomedical journals, some of which have very loose standards it seems. The journal “Biotechnology and Genetic Engineering Reviews” of the Taylor and Francis group, has just published what appears to be a clinical trial of caffeine in preterm infants. (I won’t add to the hit count of the journal by providing a direct link, but the article is listed on PubMed if you want to read the abstract: Jiang Q, Wu X. Effect of early preventive use of caffeine citrate on prevention together with treatment of BPD within premature infants and its influence on inflammatory factors. Biotechnol Genet Eng Rev. 2024;40(3):2730-44). A clinical trial in newborn infants is way outside of the apparent aims of the journal, which you can read on the website. I wonder if the journal was picked at random, or because it was known to have lax editorial standards, and/or if the article was refused in other places first. None of the named editorial board members has a medical background, (it also takes on average 209 days from submission to first decision, so stay away!) As it is not a medical journal, there is no mention of the ICMJE in the descriptions I found. It is an open-access, pay to publish journal, maybe it was cheaper than other alternatives.
Not only is this the wrong place to have published this article, it is written in very bizarre English, and has very questionable features to its design. Infants were “chosen and segregated within control and observation groups through random number table protocol” which led to two groups with “no significant difference in perioperative data” and then received caffeine by “intravenous pumping” or saline.
According to the methods, apparently “Doctors and nurses should show enough patience and love in the face of children, speak gently, smile and communicate with children, try to calm the children’s mood, so that the treatment can proceed smoothly”. Who can disagree with that?!
There is no mention of ethical review board approval.
The study was apparently performed without any funding, even though there was blood work, lab analyses, respiratory mechanics equipment and procedures, and repeated neurodevelopment testing. Several inflammatory markers (MMP-9, TNF-alpha, TLR-4) were measured prior to and after therapy. TLR-4 is of course a transmembrane receptor, so I have no idea if measuring circulating concentrations is of any interest, and the results they report are 10 fold higher than any others I have found in the literature. Also it required multiple blood samples, with the pain involved.
The authors give data for respiratory mechanics, which are almost certainly fictional, as they describe no methodology for measuring mechanics (resistance, compliance and work of breathing), which is extremely difficult in any case in spontaneously breathing newborn infants. The company that they claim made the equipment they used for lung mechanics lists no such equipment on their website.
Even more bizarrely, they report the neurodevelopment of the babies which was “evaluated by Child Development Center of China (CDCC), including Psychomotor development index (PDI) and Mental development index (MDI), with scorings ranging across 0–100 points for each part”. They give results at baseline before treatment as well as after treatment (age not specified). No actual results are given, just a p-value claiming that the caffeine group had better MDI and PDI after therapy, but not the controls. The figure of these results, figure 3, is actually the same as the figure for growth, figure 4, and shows length, head circumference and weight gain.
The desperately bad research described here makes me hope it is actually a work of fantasy, as the idea of subjecting real babies to such an awful protocol horrifies me.
A huge concern is how an apparently legitimate publisher and the editorial board of this journal can allow this garbage to pollute the medical literature. I have written to the company, Taylor and Francis, (there is apparently not currently a chief editor of the journal) and left a comment on PubPeer. If any of my gentle readers have a link with Taylor and Francis, maybe you could try to trigger a response, and hopefully a retraction, before these data get incorporated into Systematic Reviews.
I started writing this post because I wanted to discuss the new version of the Declaration of Helsinki. You will have to wait for the next issue!









😅
you don’t want to end up on the wrong end of the stick!!
Thank you Keith. I very much appreciate your advocacy on this VIP issue.
Keith, you bring up an important problem. Systematic reviews/meta-analyses tend to include everything published, no matter how obscure or dodgy the journal. As a result, poor science is given equal weight to the best work published in top journals.
There has to be a better way!!!
Pingback: Is this article trustworthy? | Neonatal Research