Extremely preterm infants become catabolic rapidly after birth, with the sudden interruption of their trans-placental nutrient supply to the fetus, who becomes a baby that has tiny stores of fat or glycogen. We progressed in neonatology from starving preterm babies in the first few days, to supplying them with just a glucose solution, to providing sick babies unable to full feed with TPN starting almost in the delivery room! This has been based on physiologic principles and the best guess of nutrient needs, and short term physiologic studies. In my NICU, we start with a solution of 3% AA in 10% dextrose, which the babies get at about 80 mL/kg/d (up to 100 if they have no arterial catheter) and so they receive amino acids at a rate equivalent to between 2.4 and 3 g/kg/d of on day 1, the next working day they have a formal TPN prescription, which will progress the amino acid amount up to 4 g/kg/d over a few days.
I have written recently about the PEPANIC trial, and have referred to trials of older children and adults, in whom early TPN after ICU admission increases complication rates, in particular hospital acquired sepsis. That is true even among adults who are considered malnourished on admission.
Extreme preterms are, of course, a different species, and we should not extrapolate any of those data to the preterm, but we can certainly learn from them.
In the ProVIDe trial, (Bloomfield FH, et al. Early Amino Acids in Extremely Preterm Infants and Neurodisability at 2 Years. N Engl J Med. 2022;387(18):1661-72), 434 ELBW infants (<1000g) who were admitted <24 hours and had a UAC placed, were randomized to either get 8.4% amino acid solution or 0.45% saline as the solution in their umbilical arterial catheter, running at 0.5 mL/h. Which would have given a 1 kg baby 1 g/kg/d more amino acids, and given a 500 g infant 2 g/kg/d of amino acids extra.

Which is the first thing I don’t understand here, the authors state that they did it this way to ensure that the intervention babies received 1 g/d more protein; the babies should, with a birth weight averaging 780g have received on average about 1.3 g/kg/d more protein in the intervention group, for as long as the UAC was in place, to a maximum of 120 hours. But, in fact, they received a supplement which was inversely proportional to their birth weight I am not sure really how relevant this is to any kind of practice that I would consider instituting. I understand the technical simplicity of designing the study this way, but surely just adding an extra 0.5 mL/kg/h of the solution would have been simple and much more clinically relevant. Then every baby would have received 1 g/kg/d extra for the intervention period.
This makes it very difficult to figure out what this means; the results showed, overall, in the intervention group, very slightly lower mortality (18% vs 19.4%, consistent with random variation) before follow up, but worse, and slightly lower, Bayley language, cognitive, and motor scores. Here below are the scores on the 3 composites, showing the numbers tested (about 93% of the surviving infants), the mean score and 1 SD, with the intervention group first, the controls second and then the adjusted mean difference with the 95% CIs.

The intervention group babies received an average of 0.8 g/kg/d of protein when calculated and averaged over the 1st week of life, but, as mentioned, that supplement will have been very variable. A 400 g baby, for example will have received 2.5 g/kg/d of additional amino acids, up to a total of 12.5 g/kg over the maximum 5 days of the study. This calculates to 1.4 g/kg/d when averaged over the 1st week. Others may have received very little; a 1kg baby having their UAC removed at 48 hours of life would have had an additional 2 g, or when calculated over the 1st week, 0.3 g/kg/d. It might seem strange to calculate the supplements over the first week, when the intervention lasted a maximum of 5 days, but the authors also did the calculations that way for the table in the supplement, which showed that all of the other nutritional intakes, of macronutrients and energy, were identical between groups.
The primary outcome was a new word, “neurodisability”, which really meant… it is not immediately obvious as it is not clearly defined in the publication, you have to download and read the protocol to be certain. To save you that extra work, I have copied the definitions below.
As is usual in studies of follow up in the very preterm infant, the majority of abnormal outcomes were due to low Bayley scores. In the control babies, 5.5% had CP, 0.6% were “blind” and 1.2% were “deaf”, therefore, most of the 37% with so-called “neurodisability” were classified as such because of a low score on one or more of the Bayley 3 composites.
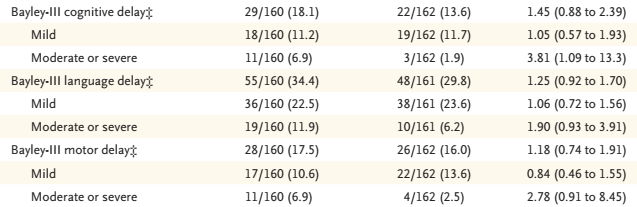
This is the part of the results table showing the proportion of tested babies with scores below 85 (“mild”), and below 70 (“moderate or severe”), on each of the composites, and the adjusted relative risk and 95% CI. You can see that most of the 95% CI included no difference, apart from the proportion with a Bayley cognitive composite <70.
The finding which is emphasized in the graphical abstract above, “of moderate to severe neurodisability” was defined posthoc: “Because few disabilities were classified as severe or moderate, these categories were combined post hoc into a single category”.
Giving additional protein, with the same amount of energy, led to an increase in serum ammonia (with 95% CI which included zero) an an increase in urea concentrations, which suggests to me that the babies weren’t utilizing all the extra protein. At the same time there was a metabolic response, as more of the intervention group babies became hypophosphataemic and hypercalaemic, which is a phenomenon that occurs after birth most frequently in babies with Intra-uterine Growth Restriction, and has been called different things in the literature, but which is analogous to the re-feeding syndrome. Babies with this occurrence often also are hypokalaemic and hypomagnesaemic, and may be hyperglycaemic, but I can’t see if those are reported in this trial.
As I mentioned above, early TPN in the PICU leads to an increase in hospital acquired sepsis, which was true for the overall group in the PEPANIC trial, and was especially true for the babies of <1 week of age. In this new trial there were no major individual changes in neonatal complications, apart from an increase in PDA needing treatment, from 42 to 54%, (aRR=1.3, 95% CI 1.05- 1.6). There was only a small increase in the proportion of babies with at least one episode of culture proven late onset sepsis, from 31 to 36% (aRR=1.19, 95% CI 0.51- 1.96). There was a small transient impact on weight gain, with the intervention groups having slightly higher body weight z-scores at 4 weeks of age, but there was no difference at discharge or at 2 years. They also performed executive function testing (BRIEF-P) and a behavioural evaluation (CBCL), which showed no striking differences.
I still don’t know, after this trial, what is the optimal amount of protein to start in the TPN of the very immature baby, and actually, I don’t think the trial helps me very much. The control babies received, when averaged over the entire first week of life, an average of 2.9 g/kg/d of protein, and about 76 kcal/kg/d. Adding somewhere between 1 and 2.5 g/kg/d for the first few days in a manner which is inversely proportional to birth weight, without changing anything else, did not have any positive impact, and there is some suggestion of a negative long term change in development. So I won’t be doing that.
I would be fascinated to see an analysis of these data by the actual amounts of extra protein received. The highest risk babies (lowest birth weight) will have received relatively more additional protein, and, if the additional protein is the cause of those potential impacts on developmental progress, then it should be more evident in those infants.
There are very few studies to put this new trial in context, in terms of large randomized trials of early nutritional interventions in the very preterm with clinical outcomes. A study from Rhode Island, (Balakrishnan M, et al. Growth and Neurodevelopmental Outcomes of Early, High-Dose Parenteral Amino Acid Intake in Very Low Birth Weight Infants: A Randomized Controlled Trial. JPEN J Parenter Enteral Nutr. 2018;42(3):597-606) enrolled 168 babies under 1250 g birth weight, to receive either 1-2 g/kg/d on day 1 increasing to 4 g/kg/d by day 5, or 3-4 g/kg/d on day 1, increasing to 4 g/kg/d by day 2. The primary outcome was from a neurological exam and developmental assessment at 2 years, and it showed no difference between groups. The Cochrane review of higher vs lower amino acid intakes of amino acids included about 20 other studies which were of various different interventions, had differing outcomes, and were all small or tiny. It showed no clear difference in any outcome.
For the future, it would be illuminating to do a similar study to ProVIDe, but to add both protein and an additional energy source, adjusted to give the same per kg supplement to all babies. That might allow better protein utilization and avoid the increase in urea concentrations, at the same time enhancing phosphorus supply, especially among the infants with IUGR, should make this safer.
Should we go lower? Is it possible that babies would have better outcomes if we started with even lower protein intakes than the ProVIDe control group? The answer is, I think, yes, it is possible, but I think we will have to be very careful, we could perhaps randomize babies to intakes which are within the range of those in current use in our NICUs, say 1.5 g/kg/d as starting dose, compared to 3 g/kg/d, with appropriate energy intakes, which also could (and probably should) be different between groups. ProVIDe, and other data, suggest that our outcomes should include PDA, late onset sepsis, and long term developmental progress. Despite the lack of very strong data to support the current practice of extremely early TPN in the very preterm, I don’t think we should return to the days of starting TPN on day 3, with just dextrose administered initially, but rather acknowledge that we are not really sure what is the optimal approach to protein and other nutrient administration in the extremely preterm infant in the first few days of life. Overall, babies do much better in terms of survival, nutritional, growth, and developmental outcomes than they have have before. We are doing somethings right, we need to fine tune to get them “righter”!








