Delayed cord clamping is now standard of care for all deliveries, at term or preterm. In term deliveries it leads to improved iron stores in the baby which difference persists during the first year of life. In very preterm infants mortality is reduced, and there is some reduction in many complications of prematurity; somewhat fewer cases of IVH, NEC, RoP (each of which, individually, are small reductions and potentially chance findings) and fewer transfusions.
Delaying clamping until pulmonary ventilation is established allows the reduction in pulmonary vascular resistance and increase in pulmonary blood flow and left heart preload to occur. It makes much physiologic sense therefore to ensure that the cord is left intact until this occurs, which complicates resuscitation in babies who don’t start breathing during the usual 60 seconds of delay in cord clamping. It is therefore common, for babies who don’t start to breathe spontaneously early, to clamp and cut the cord and take the baby to a resuscitation table to provide positive pressure ventilation.
Procedures for resuscitation with an intact cord have been developed and are being studied in preterm babies. As you might imagine this is a very difficult subject to study, especially among full term babies few of whom need to be ventilated. So bravo once again to the Melbourne group, this time to a collaborative project between the 2 centres at the Royal Women’s Hospital and Monash. (Badurdeen S, et al. Physiologically based cord clamping for infants >/=32+0 weeks gestation: A randomised clinical trial and reference percentiles for heart rate and oxygen saturation for infants >/=35+0 weeks gestation. PLoS Med. 2022;19(6):e1004029).
Even thinking about how to do such a study makes my head hurt, you need to enrol mothers at increased risk of needing neonatal resuscitation, and then randomize the babies who do actually need resuscitation to either have the usual approach (immediate clamping) or to have initial steps of resuscitation, at least attempts to establish pulmonary ventilation, with the cord intact. But very few babies who are at risk actually need positive pressure ventilation, so you have to put in place the resources required in order to screen nearly 1000 babies, enrol about 500, and finally randomize only 120 of them, as they did in this trial. Those enrolled but not randomized are babies who have delayed clamping and don’t need assisted ventilation. So the team had the great idea of collecting saturation and heart rate data from them in order to update the percentiles for normal transition, using data from infants with delayed clamping. The percentiles we all currently use are from babies with immediate clamping.
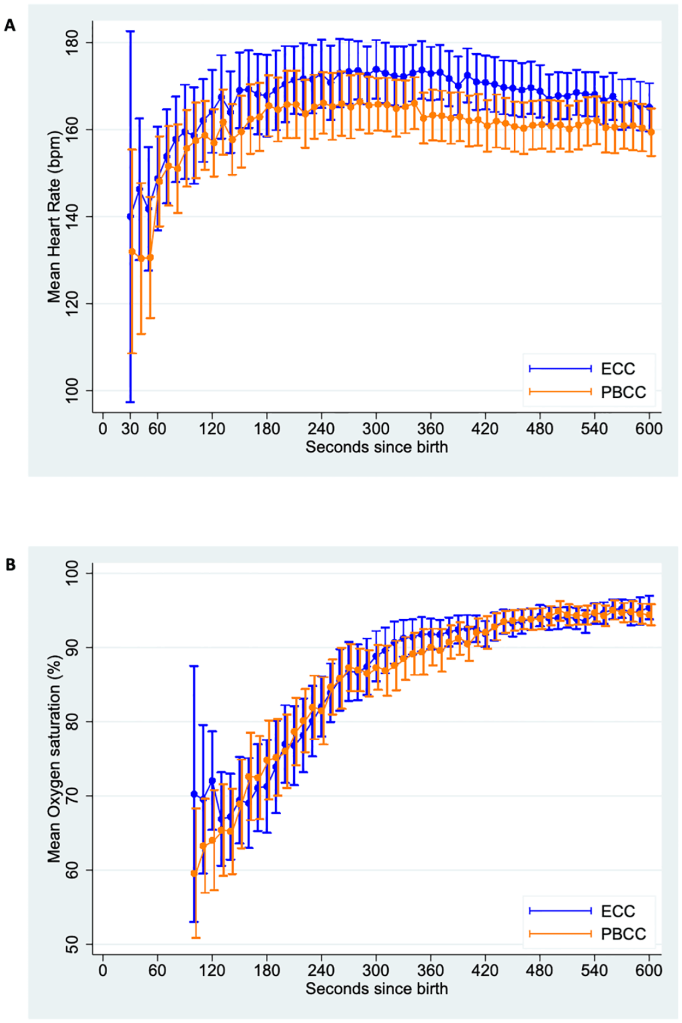
Those percentiles are here for the babies in the study of at least 35 weeks gestation who started breathing with just drying suction and stimulation who had at least 2 minutes delay before clamping the cord:

Those babies who did not start breathing when the physicians thought they needed positive pressure ventilation, in the first 60 seconds, were then randomized using a smart phone App. The randomization actually occurred at a mean time of 26 seconds after birth, and the cord was subsequently clamped in the Early cord clamping, ECC, group, at a mean of 37 seconds, and after establishing pulmonary ventilation in what they called the Physiologically based cord clamping, PBCC, group at a mean of 136 seconds.
The primary outcome for the trial was the mean heart rate between 60 and 120 seconds after birth. ECG was installed prior to 60 seconds, and the heart rate was recorded every 10 seconds between 60 to 120 s and then averaged. This is the one thing in this trial that I think is questionable, and I am sure was the subject of many discussions. I presume it was based on the findings from the animal studies, of the same group, that heart rate was lower among preterm lambs who had ECC compared to lambs with PBCC. The clinical significance of an average heart rate during this period is questionable, time to resolution of bradycardia might have been a better measure, but of course, not all the babies would be bradycardic, and further restricting the study to those with slow heart rates would have made the study unfeasible. The results show that after 30 s from birth, fewer than a third of the babies ever had a heart rate under 100. They also show that many of the randomized babies didn’t actually need to have respiratory support, 23 of the 63 PBCC babies and 32 of the 60 in the ECC group did not need any respiratory support.
The primary outcome was not different between groups, or between subgroups, and the numerous secondary outcomes were also all similar. A few of the ECC babies started breathing very quickly after randomization, either before the cord was actually clamped, or within a few seconds afterwards, and the actual heart rates were almost identical during the intervention period, and then were slightly higher in the ECC group than the PBCC.
Despite the good physiologic rationale for the practice, this study doesn’t give any support for resuscitation with an intact umbilical circulation.
In contrast a trial from Kathmandu does show a possible benefit. (Kc A, et al. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 minutes of birth – randomized clinical trial. Matern Health Neonatol Perinatol. 2019;5:7) In that trial, which was somewhat larger, and also had some of the same difficulties with design and recruitment, 1560 babies were randomized of nearly 1800 who were screened. There were 780 in each group, with 134 needing resuscitation in the delayed clamping group, and 97 with ECC.
The eligibility criteria were a bit different (>34 wks) as were procedures and outcomes; the teams were following the Helping Babies Breathe algorithms, which are slightly different in the initial steps to NRP. Babies were randomized prior to birth, but not included unless they didn’t respond to the initial steps of suctioning and stimulation and start breathing within 30 s after birth. At which time the intervention started, which was either immediate clamping, moving the baby to a resuscitation area in a room right next to the DR, or starting PPV “close to the mother in her bed” with clamping delayed at least 3 minutes. Unfortunately only half of the delayed clamping group followed the protocol, with variable durations of delayed clamping, median 105 s (IQR 30 -191). Despite this, there were differences in the primary outcome variable, which for this study was the saturation at 10 minutes of age. The mean saturation with ECC was 85%, compared to 90% for the intact cord group. There were also differences in heart rate at 1 and 5 minutes, but they were actually lower in the intact cord group than ECC, a difference of about 10 bpm at each time. The babies also cried sooner and established regular breathing more quickly. Earlier on in the resuscitation, at 1 and 5 minutes, the differences in saturation were greater between groups, 72% vs 62% at 1 minute, and 84% vs 77% at 5 minutes.
Despite the problems with following the protocol to the full 3 minutes duration of delayed clamping, it looks like most of the intact cord resuscitation group did have either positive pressure or spontaneous breathing before the cord was clamped. 66% of the delayed clamping group, and 20% of the ECC group had breathing efforts before the cord was clamped, and it looks like most of the protocol violations occurred after PPV was at least initiated. They did analyse the data only from those who had the full 3 minutes of delay in clamping, and all the heart rate and saturation differences were a bit larger between groups, but there was no real change in the spontaneous breathing outcomes compared to the ITT analysis.
The clinical importance of having saturations 5 points higher at 10 minutes is questionable, but a trial to show improved clinical outcomes would have to be enormous. Is it feasible to do such a trial? It would probably need cluster randomization, or deferred consent if individually randomized. Otherwise we would have to consent many thousands of mothers prior to delivery in order to have an adequate sample size of babies who are at risk of complications.
It is interesting that the physiologic rationale for this approach includes the demonstration in animals that clamping the cord before PPV leads to lower heart rates, whereas in this study the heart rates were higher with ECC. If you look at the animal data such as this figure from a review article
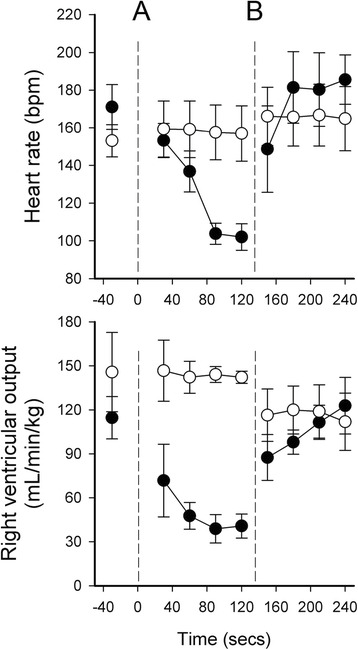
you can see that the delay in PPV after cord clamping among the lambs was over 2 minutes, and the bradycardia was not really evident for the first 30 seconds (even though RV output is very low). As we don’t wait for 2 minutes of apnoea after cord clamping in human babies, we might well not see the bradycardia that is seen in lambs, despite a probably much lower RV output.









Thanks Keith. May i ask why you continue to refer to ‘delayed’ clamping (rather than ‘deferred’ or ‘interventional’ or ‘circulatory amputation’ or something else? the language we use seems as if (a) clamping is A Good Thing and (b) ‘delayed’ must be Bad, compared to ‘early’ or ‘timely’ which we usually prefer in medicine. The language drives normalisation of an unindicated intervention. I’m struggling to think of a good reason to clamp at all, rather than have the doctor come to the baby to assess and treat it. I’m a retired obstetrician and now am ashamed of what I did in practice all those years without thinking. The only purpose (it seems to me now) was to remove the baby from the mother, often to hand it on to a neonatologist to resussitate on a faraway piece of equipment. I think we harmed babies whilst persuading ourselves we were helping. Babies ‘get’ interventions, but thats quite different from them ‘needing’ them!. How wicked is that?
Good question, words have power, and maybe we should rename the interventions. Early cord clamping could be “precocious” clamping, or your suggestion of “circulatory amputation” is an interesting idea. Later clamping could be “natural cord management” or something. We do, however, have to figure out what to do, for example, if a baby born by cesarean unexpectedly needs resuscitation. To keep the circulation intact, the resuscitation team will have to scrub in, as happened in BabyDUCC, but I don’t think that is feasible in routine practice. Maybe the best approach would be for the obstetrician to be responsible for the initial steps of resuscitation, as they are already scrubbed, and then clamp the cord and hand off to the resusc team once the baby ventilates (or is ventilated). The logistics will be important, which is one reason for doing such studies, to find out if it really matters enough to change all our practices. For deliveries where there is no need for surgical cleanliness, then it is relatively straightforward, at least if they can do it in Nepal, then we can probably figure it out in hospitals in wealthy countries too.
Thank you Keith for your excellent summary and analysis of the trial. Speaking for our Baby-DUCC team, we were excited to see the trial highlighted on your blog. Your assessment of the strengths, weaknesses, and challenges was spot on.
For what it is worth, Shiraz (1st author) and I did not find preparation or ‘scrubbing in’ for a c-section to be that difficult. I believe there is a big advantage to putting the paed into the sterile field because the the OB is then relieved of being responsible for newborn in a complex situation. Therefore, PBCC is much more likely to happen. We routinely have a NICU team member ‘scrub in’ at a c-section birth of an extremely preterm infant to place the newborn in plastic wrap and stimulate, which improves the likelihood of 60 seconds or more of DCC in my experience.
To prepare the equipment for Baby DUCC, we sent the non-sterile items for sterilisation via autoclave (onsite at the two participating hospitals) or for ethylene oxide sterilisation (offsite), which is standard practice for all the equipment used in a sterile field in Melbourne. The clinicians involved in the trial were mostly registrars and NNPs who were at the births were participating in Baby DUCC for the first time and they handled the extra logistics without issues. If we had a reg or NNP for a second or third birth, they were old pros. If PBCC becomes a standard approach, the preparation of the equipment would become routine, which would likely include using purpose built platforms like the Lifestart trolly or the CONCORD (used in the ABC3 trial). We intentionally designed the trial to use items already available in our hospitals to show that that PBCC works with a low-tech approach. Although we didn’t show an advantage based on our outcomes, we did show good adherence to the protocol and provide some encouraging safety data. Again, thanks for highlighting our work, we really appreciate it!
Thanks Keith. Why would a baby ‘unexpectedly’ need resuscitation after a CS and how long would it take to assess matters? Yes, I think we should make obstetricians more confident and competent, but they first have to realise that clamping is a harm – and they don’t relieve themselves of responsibility by doing it – maybe they make matters worse. I am not confident that the ‘standard of care’ is being adhered to – where is the evidence that people are waiting at least a minute if not ‘waiting ’till white’? I came from an era of having done 6/12 in a neonatal unit early in my career so was more confident that most obstetricians that the baby was well, transitioning and not needing to be passed to someone else to take away the responsibility. Maybe a neonatologist needs to scrub in. There are ‘operating table’-side resuscitation platforms, and it cant be beyond the wit of man to devise a way of passing the baby to the non-sterile and mothers side of the drapes to be assessed/ aided. Maybe if we hadn’t damaged so many babies’ transition, or make the slightly unwell worse people wouldnt be so frightened of vaginal birth in the first place (where there are less ‘experts’ fighting for space…!!