When the platypus was first captured, killed, skinned and the pelt sent to a zoologist in London, George Shaw in 1799, he thought he was being scammed. He tried to find the stitches that had been used to cobble together the specimen. Being unable to do so, he then concluded that it was probably real, and was unique. It was what we would now consider an evolutionary innovation, it had features of previous organisms, such as laying eggs, and several new features, being venomous and producing milk to feed their young, once they have hatched. Here is a photo I took in March 2023 at the Woonoowooran national park.

Brett Manley and the group from Melbourne are doing something analogous with their PLATIPUS (Manley BJ, et al. Adapt to survive and thrive: the time is now for adaptive platform trials for preterm birth. Lancet Child Adolesc Health. 2025;9(2):131-7) adaptive platform. Taking features of standard RCTs, and evolving a new way to do trials in perinatal medicine.
Adaptive designs first went mainstream during the COVID epidemic, with the dramatically effective RECOVERY platform. The trials were able to be launched, and to rapidly provide clinically vital results within a very short period of time, while the pandemic was still at its height. RECOVERY was able to rapidly prove the value of standard dose steroid treatment for improving outcomes, and then showed that higher dose dexamethasone was a bad idea, and should not be used. They have also shown that some antivirals are ineffective, that routine azithromycin made no difference, and have to this point enrolled nearly 50,000 patients, having now expanded to find treatments for complicated influenza and non-viral community acquired pneumonia.
Another platform design has, just this last week, produced some important results in ALS, also known as Lou Gehrig’s disease in the USA, and Motor Neurone Disease in the UK. There are 4 simultaneous comparisons published, with 3 different interventions compared to a common control group. None of the interventions showed a clear benefit, but there were some suggestions of some improvements, which allows, within the platform design, to proceed rapidly to adjusted protocols, perhaps in subgroups. An editorial article accompanying the publications discusses the limitations, and the major advantages of such trials. In ALS, the Healey platform was funded by, and named after, a very wealthy man who has the condition himself.
The first comparisons being investigated by PLATIPUS are, for the maternal domain, different antibiotic regimens for preterm PROM, with 3 different regimens being investigated. I must say I thought this was relatively settled, among the antibiotics in the trial, and that it was already fairly clear that azithromycin plus amoxycillin was the way to go, but it seems there is still some doubt about their relative efficacy, and the platform will allow the future investigation of other regimes with minimal adjustment. For the neonatal domain of the platform, they are investigating 3 different dosage regimes of caffeine, the standard dose that we used in the CAP trial, (20 mg/kg of caffeine citrate load, then 10 mg/kg/d, which they call the low-dose group) to a medium dose group which is 50% higher, and a high dose group which is double the standard dose.
The figure below is a schematic of how it might proceed.
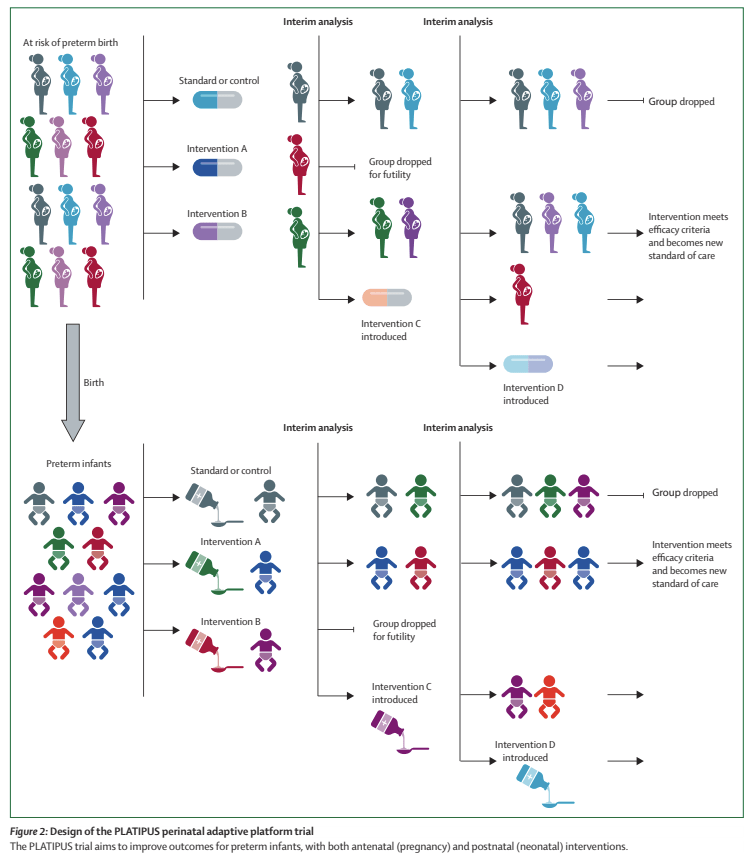
The other part of the innovative nature of this trial is the outcome measure that is being used, which is an ordinal composite adjudicated at 42 weeks PMA. The outcome is scored from 1 to 15, with 15 being the worst outcome, death. Levels 1 to 15 are described as follows:
1= Well, liveborn infant; 2= Neonatal unit admission for <48 hours; 3= Neonatal unit admission for >/= 48 hours; 4= Non-invasive respiratory support or oxygen therapy for ≥ 4 hours & < 5 days; 5= Non-invasive respiratory support or oxygen therapy >/= 5 days; 6= Mechanical ventilation via endotracheal tube for ≥ 4 hours & <7 days; 7= Mechanical ventilation via endotracheal tube for >/=7 days; 8= Moderate respiratory morbidity; 9=Necrotising enterocolitis AND/OR Sepsis; 10= Severe Respiratory Morbidity; 11= Major Surgery; 12= Brain Injury; 13= TWO of severe respiratory morbidity OR major surgery OR brain injury; 14= Severe respiratory morbidity & major surgery & brain injury; 15 = Death
There is obviously a lot to pick apart and discuss here, but my first response is Bravo!! At last a composite outcome in a neonatal trial that recognizes that all competing outcomes do not have the same value! The definitions of each of these outcomes are on the clinicaltrials.gov website. For example “severe respiratory morbidity” is respiratory support at 40 weeks PMA, or discharge home on oxygen or respiratory support. Which seems to me to be a definition which is both consistent with parental concerns and reasonably predictive of longer term respiratory outcomes.
As for Brain Injury, I have a few more concerns, the definition given is : Major intraventricular haemorrhage, unilateral or bilateral, defined as i. Papile Grade 3 or 4 AND/OR ii. Moderate-severe periventricular haemorrhagic infarction b. Cystic periventricular leukomalacia, unilateral or bilateral c. Moderate or severe white matter injury on MRI at near-term or term equivalent age d. Any cerebellar haemorrhage e. Other major ischaemic injury such as arterial stroke or hypoxic ischaemic injury f. Post haemorrhagic hydrocephalus requiring drainage.
The problems with this part of the outcome are 1. using the Papile classification 2. including “any” cerebellar haemorrhage. 3. White matter injury on the MRI.
- 1. The Papile classification has numerous problems, specifically for this definition the definition of a grade 3 haemorrhage is unclear, and some babies with a grade 2 haemorrhage and some early, often transient, ventricular dilatation may get reclassified as having a grade 3 bleed. Grade 4 haemorrhages are also very variable, and a small unilateral intracerebral bleed may have very little impact on long term outcomes, quite different to extensive bilateral haemorrhage. We discussed a lot of the prognostic data in our publication : Chevallier M, et al. Decision-making for extremely preterm infants with severe hemorrhages on head ultrasound: Science, values, and communication skills. Semin Fetal Neonatal Med. 2023;28(3):101444.
- 2. Cerebellar bleeds, also, may be punctate and have little long term implication, or may be more extensive and problematic.
- 3. White matter injury on near-term MRI has very low individual prognostic value for future outcomes.
For this particular part of the composite outcome, I am not sure how much this matters, but if the high-dose caffeine group, for example, had more cerebellar haemorrhages, but less severe respiratory morbidity, then that group would be considered to be worse off, even if the haemorrhages were all tiny and had no long term impact. However, I am not sure if you could really tease all that out as part of an ordinal composite like this one, if there is a difference in brain injury, as defined in the protocol, between groups, that would probably be a reason for choosing between interventions, even if some of the haemorrhages had little long term impact.
For an adaptive platform trial trial like this the outcomes have to be determined rapidly after the intervention; 2 year neurological/developmental outcomes, or other long term outcomes of interest to parents cannot be the primary. Hopefully, all the infants randomized in the platform will have longer term outcomes evaluated and reported in addition to the primary short term outcome. I’m sure you are all aware that caffeine therapy in the CAP trial did have some minor short term benefits (infants came out of oxygen a little faster, so were less likely to be diagnosed with BPD, and less likely to have treatment for a PDA), but substantial, very long lasting benefits on follow-up.
Analysis of the trial results is also a major issue, the RECOVERY trials have used a fairly standard frequentist approach, with the first results showing advantages of standard dose dexamethasone being presented as the proportion of deaths in each group, the relative risk (0.83) and confidence intervals (0.75 to 0.93) and the p-value P<0.001. Platipus is planned with a Bayesian approach, which is less familiar to many clinicians, but has potential advantages. The Bayesian approach clarifies that no RCT really proves that one approach is better than another, which is suggested by our dichotomous “significant or not significant” current typical presentation of results, but gives an estimate of how likely it is that one intervention is better than another.
The Platipus platform will allow on-going adjustments, other comparisons, addition of other groups, and other interventions. It could become the default model for future trials in perinatal medicine, and should enable us to get results much faster, but just as reliably.
Platipus, just like the Platypus, will give us major scientific insights for the future. It should allow us to advance the care of newborns more rapidly than in the past.









Hi Keith, thanks for looking at PLATIPUS. I really enjoyed your commentary. The ordinal outcome has been debated a lot and there are certainly pros and cons with some items. We wanted something that would cover the whole spectrum of preterm birth. We hope to have publications soon giving more background on the primary outcome and Bayesian approach. There is a PLATIPUS Follow-up Group working on best ways to address long-term outcomes at scale. http://www.platipus.org
Thanks for the commentary, Keith. An enjoyable read.
I’m not sure we realised the enormity of the task we set ourselves when we started developing this ordinal outcome. Many of the points you raise have been discussed at length by the PLATIPUS group and collaborators. In the end we’ve tried to be pragmatic and use outcomes/definitions that are easily measured and important.
Your commentary prompted us re-visit our ‘Brain Injury’ definitions.
These aren’t perfect definitions. And the ordinal isn’t perfect – I expect we’ll learn more as we begin to use it, and the ordinal will evolve. But still reckon it’s a better outcome measure than ‘Death or BPD’…
Thanks for these clarifications, which address just about everything that was a concern. I don’t think there is any way to make this perfect, and we should recognise that different items may well have different value for different families. For a platform such as this, the outcomes have to be short term, and therefore will not reflect everything that is of importance in the long-term, but the major advantages of having a flexible, on-going, platform for future comparisons are huge.
I have no idea how I would do this myself! For example, should small, unilateral intracerebral haemorrhages (Bassan score of 1) be classified along with grade 2s, or should we decide that probably anything that reduces small grade 4s will likely also reduce large grade 4s, and therefore keep all the grade 4s together, even while recognizing that some are much more predictive of the long term than others.
I am so glad that your team has taken this on, with a thoughtful group like yours developing the platform we will benefit from some really important data in the future. Maybe the platform can be expanded to include other parts of the world.